| |
| Names | |
|---|---|
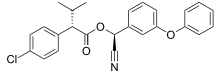
| Preferred IUPAC name (S)-Cyano(3-phenoxyphenyl)methyl (2S)-2-(4-chlorophenyl)-3-methylbutanoate | |
| Other names
Asana (S)-Fenvalerate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 4275674 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.118.804 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| UNII | |
| UN number | 3349 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C25H22ClNO3 |
| Molar mass | 419.91 g·mol |
| Density | 1.211 g/cm |
| Melting point | 60 °C (140 °F; 333 K) |
| log P | 6.22 |
| Vapor pressure | 0 mmHg at 25 °C |
| Hazards | |
| GHS labelling: | |
| Pictograms |    
|
| Signal word | Danger |
| Hazard statements | H301, H313, H316, H317, H320, H330, H335, H370, H373, H410 |
| Precautionary statements | P260, P261, P264, P270, P271, P272, P273, P280, P284, P301+P310, P302+P352, P304+P340, P305+P351+P338, P307+P311, P310, P312, P314, P320, P321, P330, P332+P313, P333+P313, P337+P313, P363, P391, P403+P233, P405, P501 |
| Flash point | 256 °C (493 °F; 529 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Esfenvalerate is a synthetic pyrethroid insecticide marketed under the brand Asana. It is the (S)-enantiomer of fenvalerate.
In the United States, a limit of .05 ppm of the chemical's residue is permissible in food.
References
- Kelly, Kevin. "Environmental Fate of Esfenvalerate". California Environmental Protection Agency. Retrieved January 10, 2013.
- Fishel, Frederick M. (2012). "Pesticide Toxicity Profile: Synthetic Pyrethroid Pesticides". University of Florida. Archived from the original on May 12, 2016. Retrieved January 10, 2013.
- "Esfenvalerate". EXTONET (Extension Toxicology Network). Cooperative Extension Offices of Cornell University, Michigan State University, Oregon State University, and University of California at Davis. May 1994. Retrieved January 10, 2013.
- The Code of Federal Regulations of the United States of America. U.S. Government Printing Office. 2006. pp. 445–446.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |