Pharmaceutical compound
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Palladia |
| AHFS/Drugs.com | Veterinary Use |
| License data | |
| Routes of administration | By mouth |
| Drug class | Antineoplastic agent |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 77% |
| Protein binding | 91%-93% |
| Elimination half-life | 16 h |
| Identifiers | |
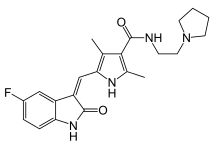
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL |
|
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H25FN4O2 |
| Molar mass | 396.466 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Toceranib (INN), sold under the brand name Palladia, is a receptor tyrosine kinase inhibitor that is used in the treatment of canine mast cell tumor also called mastocytoma. It is the first medication developed specifically for the treatment of cancer in dogs. It is used as its phosphate salt, toceranib phosphate. It was developed by SUGEN as SU11654, a sister compound to sunitinib, which was later approved for human therapies. Toceranib is a tyrosine kinase inhibitor and works in two ways: by killing tumor cells and by cutting off the blood supply to the tumor.
The most common side effects include diarrhea, decrease or loss of appetite, lameness, weight loss, and blood in the stool.
Veterinary uses
Toceranib is indicated to treat canine cutaneous (skin-based) mast cell tumors, a type of cancer responsible for about one out of five cases of canine skin tumors. It is approved to treat the tumors with or without regional lymph node involvement.
References
- "Palladia EPAR". European Medicines Agency. 1 October 2009. Retrieved 1 July 2024.
- World Health Organization (2009). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 62". WHO Drug Information. 23 (2). hdl:10665/74420.
- London CA, Malpas PB, Wood-Follis SL, Boucher JF, Rusk AW, Rosenberg MP, et al. (June 2009). "Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision". Clinical Cancer Research. 15 (11): 3856–3865. doi:10.1158/1078-0432.CCR-08-1860. PMID 19470739.
- ^ "FDA: First Drug to Treat Cancer in Dogs Approved". U.S. Food and Drug Administration (FDA) (Press release). 3 June 2009. Archived from the original on 22 July 2010. Retrieved 2 October 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- "Palladia New Animal Drug Application" (PDF). U.S. Food and Drug Administration (FDA). 22 May 2009. Archived from the original (PDF) on 16 November 2010. Retrieved 2 October 2021.
- "In Trials for New Cancer Drugs, Family Pets Are Benefiting, Too". The New York Times. 24 November 2006. Archived from the original on 27 February 2021. Retrieved 2 October 2021.
| Growth factor receptor modulators | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiopoietin |
| ||||||||||
| CNTF |
| ||||||||||
| EGF (ErbB) |
| ||||||||||
| FGF |
| ||||||||||
| HGF (c-Met) |
| ||||||||||
| IGF |
| ||||||||||
| LNGF (p75) |
| ||||||||||
| PDGF |
| ||||||||||
| RET (GFL) |
| ||||||||||
| SCF (c-Kit) |
| ||||||||||
| TGFβ |
| ||||||||||
| Trk |
| ||||||||||
| VEGF |
| ||||||||||
| Others |
| ||||||||||
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |
This veterinary medicine–related article is a stub. You can help Misplaced Pages by expanding it. |