| Revision as of 22:02, 17 April 2024 editBeland (talk | contribs)Autopatrolled, Administrators237,091 editsm convert special characters found by Misplaced Pages:Typo Team/moss (via WP:JWB)← Previous edit | Latest revision as of 21:13, 24 December 2024 edit undoHaley275 (talk | contribs)Extended confirmed users724 edits →Safety: Added a brief subsection (3 sentences) indicating that uranyl acetate can induce cellular DNA damage. | ||
| Line 88: | Line 88: | ||
| Microbiologists have developed a number of alternative ]:<ref>Yamaguchi K, Suzuki K, Tanaka K (2010) Examination of electron stains as a substitute for uranyl acetate for the ultrathin sections of bacterial cells. J Electron Microsc (Tokyo) 59:113–118</ref> ],<ref name="NdAc-UOAc alt">{{cite journal |last1=Kuipers |first1=Jeroen |last2=Giepmans |first2=Ben N. G. |title=Neodymium as an alternative contrast for uranium in electron microscopy |journal=Histochemistry and Cell Biology |date=1 April 2020 |volume=153 |issue=4 |pages=271–277 |doi=10.1007/s00418-020-01846-0 |pmid=32008069 |pmc=7160090 |language=en |issn=1432-119X}}</ref><ref>Hosogi N, Nishioka H, Nakakoshi M (2015) Evaluation of lanthanide salts as alternative stains to uranyl acetate. Microscopy (Oxf) 64:429–435</ref> platinum blue,<ref>Inaga S, Katsumoto T, Tanaka K, Kameie T, Nakane H, Naguro T (2007) Platinum blue as an alternative to uranyl acetate for staining in transmission electron microscopy. Arch Histol Cytol 70:43–49</ref> hafnium chloride,<ref>Ikeda K, Inoue K, Kanematsu S, Horiuchi Y, Park P (2011) Enhanced effects of nonisotopic hafnium chloride in methanol as a substitute for uranyl acetate in TEM contrast of ultrastructure of fungal and plant cells. Microsc Res Tech 74:825–830</ref> and ] tea extracts.<ref>Sato S, Adachi A, Sasaki Y, Ghazizadeh M (2008) Oolong tea extract as a substitute for uranyl acetate in staining of ultrathin sections. J Microsc 229:17–20</ref><ref>He X, Liu B (2017) Oolong tea extract as a substitute for uranyl acetate in staining of ultrathin sections based on examples of animal tissues for transmission electron microscopy. J Microsc 267:27–33</ref> | Microbiologists have developed a number of alternative ]:<ref>Yamaguchi K, Suzuki K, Tanaka K (2010) Examination of electron stains as a substitute for uranyl acetate for the ultrathin sections of bacterial cells. J Electron Microsc (Tokyo) 59:113–118</ref> ],<ref name="NdAc-UOAc alt">{{cite journal |last1=Kuipers |first1=Jeroen |last2=Giepmans |first2=Ben N. G. |title=Neodymium as an alternative contrast for uranium in electron microscopy |journal=Histochemistry and Cell Biology |date=1 April 2020 |volume=153 |issue=4 |pages=271–277 |doi=10.1007/s00418-020-01846-0 |pmid=32008069 |pmc=7160090 |language=en |issn=1432-119X}}</ref><ref>Hosogi N, Nishioka H, Nakakoshi M (2015) Evaluation of lanthanide salts as alternative stains to uranyl acetate. Microscopy (Oxf) 64:429–435</ref> platinum blue,<ref>Inaga S, Katsumoto T, Tanaka K, Kameie T, Nakane H, Naguro T (2007) Platinum blue as an alternative to uranyl acetate for staining in transmission electron microscopy. Arch Histol Cytol 70:43–49</ref> hafnium chloride,<ref>Ikeda K, Inoue K, Kanematsu S, Horiuchi Y, Park P (2011) Enhanced effects of nonisotopic hafnium chloride in methanol as a substitute for uranyl acetate in TEM contrast of ultrastructure of fungal and plant cells. Microsc Res Tech 74:825–830</ref> and ] tea extracts.<ref>Sato S, Adachi A, Sasaki Y, Ghazizadeh M (2008) Oolong tea extract as a substitute for uranyl acetate in staining of ultrathin sections. J Microsc 229:17–20</ref><ref>He X, Liu B (2017) Oolong tea extract as a substitute for uranyl acetate in staining of ultrathin sections based on examples of animal tissues for transmission electron microscopy. J Microsc 267:27–33</ref> | ||
| ===DNA damage=== | |||
| Uranyl acetate can enter ] where it tends to localize in the nucleus.<ref name = Yellowhair2018>{{cite journal |vauthors=Yellowhair M, Romanotto MR, Stearns DM, Clark Lantz R |title=Uranyl acetate induced DNA single strand breaks and AP sites in Chinese hamster ovary cells |journal=Toxicol Appl Pharmacol |volume=349 |issue= |pages=29–38 |date=June 2018 |pmid=29698738 |pmc=5972677 |doi=10.1016/j.taap.2018.04.022 |url=}}</ref> Uranyl acetate can then induce ]s and ]s in the CHO cells.<ref>{{cite journal |vauthors=Stearns DM, Yazzie M, Bradley AS, Coryell VH, Shelley JT, Ashby A, Asplund CS, Lantz RC |title=Uranyl acetate induces hprt mutations and uranium-DNA adducts in Chinese hamster ovary EM9 cells |journal=Mutagenesis |volume=20 |issue=6 |pages=417–23 |date=November 2005 |pmid=16195314 |doi=10.1093/mutage/gei056 |url=}}</ref> Also in CHO cells uranyl acetate can interact with DNA to induce single-strand breaks and apurinic(apyrimidinic) sites.<ref name = Yellowhair2018/> | |||
| ==References== | ==References== | ||
Latest revision as of 21:13, 24 December 2024

| |
 Hydrated crystal structure | |
| Names | |
|---|---|
| IUPAC name Uranium bis((acetato)-O)dioxo-dihydrate | |
| Other names Uranyl ethanoate; Uranyl acetate dihydrate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.971 |
| EC Number |
|
| PubChem CID |
|
| UNII |
|
| CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | UO2(CH3COO)2 (anhydrous) UO2(CH3COO)2·2H2O (dihydrate) |
| Molar mass | 424.146 g/mol (dihydrate) |
| Appearance | yellow-green crystals (dihydrate) |
| Density | 2.89 g/cm (dihydrate) |
| Melting point | decomposes at 80 °C (dihydrate) |
| Solubility in water | 7-8 g/100 ml |
| Solubility | slightly soluble in ethanol |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H300, H330, H373, H411 |
| Precautionary statements | P260, P264, P270, P271, P273, P284, P301+P310, P304+P340, P310, P314, P320, P321, P330, P391, P403+P233, P405, P501 |
| Safety data sheet (SDS) | External MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
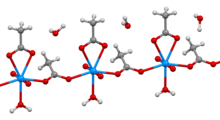
Uranyl acetate is the acetate salt of uranium oxide, a toxic yellow-green powder useful in certain laboratory tests. Structurally, it is a coordination polymer with formula UO2(CH3CO2)2(H2O)·H2O.
Structure
In the polymer, uranyl (UO2) centers are bridged by acetate ligands. The remainder of each (heptacoordinate) coordination sphere is provided by an aquo ligand and a bidentate acetate ligand. One water of crystallization occupies the lattice.
Uses
Uranyl acetate is extensively used as a negative stain in electron microscopy. Most procedures in electron microscopy for biology require the use of uranyl acetate. Negative staining protocols typically treat the sample with 1% to 5% aqueous solution. Uranyl acetate staining is simple and quick to perform and one can examine the sample within a few minutes after staining. Some biological samples are not amenable to uranyl acetate staining and, in these cases, alternative staining techniques and or low-voltage electron microscopy technique may be more suitable.
1% and 2% uranyl acetate solutions are used as an indicator, and a titrant in stronger concentrations in analytical chemistry, as it forms an insoluble salt with sodium (the vast majority of sodium salts are water-soluble). Uranyl acetate solutions show evidence of being sensitive to light, especially UV, and will precipitate if exposed.
Uranyl acetate is also used in a standard test—American Association of State Highway and Transportation Officials (AASHTO) Designation T 299—for alkali-silica reactivity in aggregates (crushed stone or gravel) being considered for use in cement concrete.
Uranyl acetate dihydrate has been used as a starting reagent in experimental inorganic chemistry.
Related compounds
Uranyl carboxylates are known for diverse carboxylic acids (formate, butyrate, acrylate).
Safety
Uranyl acetate is both chemically toxic and mildly radioactive. Chronic-exposure effects may cumulate.
In general, uranium salts exhibit nephrotoxicity. Normal commercial stocks from depleted uranium have typical specific activity 0.37–0.51 microcuries per gram (14–19 kBq/g), too weak to harm from outside the body. However, uranyl acetate is very toxic if ingested, inhaled as dust, or absorbed through cut or abraded skin.
Microbiologists have developed a number of alternative stains: neodymium acetate, platinum blue, hafnium chloride, and oolong tea extracts.
DNA damage
Uranyl acetate can enter Chinese hamster ovary (CHO) cells where it tends to localize in the nucleus. Uranyl acetate can then induce DNA adducts and mutations in the CHO cells. Also in CHO cells uranyl acetate can interact with DNA to induce single-strand breaks and apurinic(apyrimidinic) sites.
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 3–566, ISBN 0-8493-0594-2
- Howatson, J.; Grev, D.M.; Morosin, B. (1975). "Crystal and molecular structure of uranyl acetate dihydrate". Journal of Inorganic and Nuclear Chemistry. 37 (9): 1933–1935. doi:10.1016/0022-1902(75)80918-3.
- "Negative Staining" University of Oxford
- Sessler, Jonathan L.; Seidel, Daniel; Vivian, Anne E.; Lynch, Vincent; Scott, Brian L.; Keogh, D. Webster (2001). "Hexaphyrin(1.0.1.0.0.0): An Expanded Porphyrin Ligand for the Actinide Cations Uranyl (UO2) and Neptunyl (NpO2)". Angewandte Chemie International Edition. 40 (3): 591–594. doi:10.1002/1521-3773(20010202)40:3<591::AID-ANIE591>3.0.CO;2-0.
- Klepov, Vladislav V.; Vologzhanina, Anna V.; Alekseev, Evgeny V.; Pushkin, Denis V.; Serezhkina, Larisa B.; Sergeeva, Olga A.; Knyazev, Aleksandr V.; Serezhkin, Viktor N. (2016). "Structural diversity of uranyl acrylates". CrystEngComm. 18 (10): 1723–1731. doi:10.1039/C5CE01957E.
- Yamaguchi K, Suzuki K, Tanaka K (2010) Examination of electron stains as a substitute for uranyl acetate for the ultrathin sections of bacterial cells. J Electron Microsc (Tokyo) 59:113–118
- Kuipers, Jeroen; Giepmans, Ben N. G. (1 April 2020). "Neodymium as an alternative contrast for uranium in electron microscopy". Histochemistry and Cell Biology. 153 (4): 271–277. doi:10.1007/s00418-020-01846-0. ISSN 1432-119X. PMC 7160090. PMID 32008069.
- Hosogi N, Nishioka H, Nakakoshi M (2015) Evaluation of lanthanide salts as alternative stains to uranyl acetate. Microscopy (Oxf) 64:429–435
- Inaga S, Katsumoto T, Tanaka K, Kameie T, Nakane H, Naguro T (2007) Platinum blue as an alternative to uranyl acetate for staining in transmission electron microscopy. Arch Histol Cytol 70:43–49
- Ikeda K, Inoue K, Kanematsu S, Horiuchi Y, Park P (2011) Enhanced effects of nonisotopic hafnium chloride in methanol as a substitute for uranyl acetate in TEM contrast of ultrastructure of fungal and plant cells. Microsc Res Tech 74:825–830
- Sato S, Adachi A, Sasaki Y, Ghazizadeh M (2008) Oolong tea extract as a substitute for uranyl acetate in staining of ultrathin sections. J Microsc 229:17–20
- He X, Liu B (2017) Oolong tea extract as a substitute for uranyl acetate in staining of ultrathin sections based on examples of animal tissues for transmission electron microscopy. J Microsc 267:27–33
- ^ Yellowhair M, Romanotto MR, Stearns DM, Clark Lantz R (June 2018). "Uranyl acetate induced DNA single strand breaks and AP sites in Chinese hamster ovary cells". Toxicol Appl Pharmacol. 349: 29–38. doi:10.1016/j.taap.2018.04.022. PMC 5972677. PMID 29698738.
- Stearns DM, Yazzie M, Bradley AS, Coryell VH, Shelley JT, Ashby A, Asplund CS, Lantz RC (November 2005). "Uranyl acetate induces hprt mutations and uranium-DNA adducts in Chinese hamster ovary EM9 cells". Mutagenesis. 20 (6): 417–23. doi:10.1093/mutage/gei056. PMID 16195314.
| Uranium compounds | |||
|---|---|---|---|
| U(II) | |||
| U(III) |
| ||
| U(IV) |
| ||
| U(IV,V) | |||
| U(IV,VI) | |||
| U(V) | |||
| U(VI) | |||
| U(XII) |
| ||
| Acetyl halides and salts of the acetate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||