| Revision as of 19:20, 18 May 2011 edit128.172.94.70 (talk) →Side effects← Previous edit | Latest revision as of 03:59, 30 September 2024 edit undoWhywhenwhohow (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers49,185 edits rank; EML | ||
| (221 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Anti-hypertension medication}} | |||
| {{drugbox | verifiedrevid = 418536931 | |||
| {{Distinguish|hydrazine}} | |||
| | | |||
| {{Use dmy dates|date=October 2022}} | |||
| | IUPAC_name = 1-hydrazinylphthalazine | |||
| {{Drugbox | |||
| | verifiedrevid = 443859600 | |||
| | image = Hydralazine.svg | | image = Hydralazine.svg | ||
| | alt = Skeletal formula of hydralazine | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | width = 125 | |||
| | image2 = Hydralazine-based-on-xtals-3D-bs-17.png | |||
| | alt2 = Ball-and-stick model of the hydralazine molecule | |||
| <!-- Clinical data --> | |||
| | tradename = Apresoline, BiDil, others | |||
| | Drugs.com = {{drugs.com|monograph|hydralazine-hydrochloride}} | |||
| | MedlinePlus = a682246 | |||
| | DailyMedID = Hydralazine | |||
| | pregnancy_AU = C | |||
| | routes_of_administration = ], ] | |||
| | ATC_prefix = C02 | |||
| | ATC_suffix = DB02 | |||
| | legal_AU = S4 | |||
| | legal_AU_comment = <ref>{{cite web | title=Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017 | website=Therapeutic Goods Administration (TGA) | date=21 June 2022 | url=https://www.tga.gov.au/resources/publication/publications/prescription-medicines-registration-new-generic-medicines-and-biosimilar-medicines-2017 | access-date=30 March 2024}}</ref> | |||
| | legal_CA = Rx-only | |||
| | legal_UK = POM | |||
| | legal_US = Rx-only | |||
| <!-- Pharmacokinetic data --> | |||
| | bioavailability = 26–50% | |||
| | protein_bound = 85–90% | |||
| | metabolism = ] | |||
| | elimination_half-life = 2–8 hours, 7–16 hours (renal impairment) | |||
| | excretion = ] | |||
| | onset = 5 to 30 min<ref name=AHFS2016/> | |||
| | duration_of_action = 2 to 6 hrs<ref name=AHFS2016/> | |||
| <!-- Identifiers --> | |||
| | CAS_number_Ref = {{cascite|correct|??}} | |||
| | CAS_number = 86-54-4 | |||
| | PubChem = 3637 | |||
| | IUPHAR_ligand = 7326 | |||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | DrugBank = DB01275 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChemSpiderID = 3511 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = 26NAK24LS8 | | UNII = 26NAK24LS8 | ||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | InChI = 1/C8H8N4/c9-11-8-7-4-2-1-3-6(7)5-10-12-8/h1-5H,9H2,(H,11,12) | |||
| | KEGG = D08044 | |||
| | smiles = n2nc(c1ccccc1c2)NN | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | InChIKey = RPTUSVTUFVMDQK-UHFFFAOYAB | |||
| | ChEBI = 5775 | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | ChEMBL = 276832 | | ChEMBL = 276832 | ||
| <!--Chemical data--> | |||
| | IUPAC_name = 1-hydrazinylphthalazine | |||
| | C=8 | H=8 | N=4 | |||
| | smiles = NNc1c2ccccc2cnn1 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C8H8N4/c9-11-8-7-4-2-1-3-6(7)5-10-12-8/h1-5H,9H2,(H,11,12) | | StdInChI = 1S/C8H8N4/c9-11-8-7-4-2-1-3-6(7)5-10-12-8/h1-5H,9H2,(H,11,12) | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = RPTUSVTUFVMDQK-UHFFFAOYSA-N | | StdInChIKey = RPTUSVTUFVMDQK-UHFFFAOYSA-N | ||
| | CAS_number = 86-54-4 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChemSpiderID = 3511 | |||
| | ATC_prefix = C02 | |||
| | ATC_suffix = DB02 | |||
| | PubChem = 3637 | |||
| | DrugBank = | |||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | KEGG = D08044 | |||
| | C=8 | H=8 | N=4 | |||
| | molecular_weight = 160.176 g/mol | |||
| | bioavailability = 26-55% | |||
| | protein_bound = | |||
| | metabolism = Hepatic | |||
| | elimination_half-life = 2-4 hours | |||
| | excretion = Renal | |||
| | pregnancy_category = C<br/>Commonly used to treat severe ] | |||
| | legal_status = | |||
| | routes_of_administration = Oral, ] | |||
| }} | }} | ||
| <!-- Definition and medical uses --> | |||
| '''Hydralazine''', sold under the brand name '''Apresoline''' among others, is a medication used to treat ] and ].<ref name=AHFS2016>{{cite web|title=Hydralazine Hydrochloride|url=https://www.drugs.com/monograph/hydralazine-hydrochloride.html|publisher=The American Society of Health-System Pharmacists|access-date=8 December 2016|url-status=live|archive-url=https://web.archive.org/web/20161221010517/https://www.drugs.com/monograph/hydralazine-hydrochloride.html|archive-date=21 December 2016}}</ref> This includes ] and ].<ref name=WHO2008/> It has been found to be particularly useful in heart failure, together with ], for treatment of ].<ref name=AHFS2016/> It is given by mouth or ].<ref name=WHO2008>{{cite book | title = WHO Model Formulary 2008 | year = 2009 | isbn = 9789241547659 | vauthors = ((World Health Organization)) | veditors = Stuart MC, Kouimtzi M, Hill SR | hdl = 10665/44053 | author-link = World Health Organization | publisher = World Health Organization | hdl-access=free |page=280}}</ref> Effects usually begin around 15 minutes and last up to six hours.<ref name=AHFS2016/> | |||
| <!-- Side effects and mechanism --> | |||
| '''Hydralazine''' ('''Apresoline''') is a direct-acting ] relaxant used to treat ] by acting as a ] primarily in ] and ]. By relaxing ], vasodilators act to decrease ], thereby lowering ] and decreasing afterload.<ref name=Harvey>Harvey, Richard A., Pamela A. Harvey, and Mark J. Mycek. Lippincott's Illustrated Reviews: Pharmacology. 2nd ed. Philadelphia: Lipincott, Williams & Wilkins, 2000. 190.</ref> | |||
| Common side effects include ] and ].<ref name=AHFS2016/> It is not recommended in people with ] or in those with ] that affects the ].<ref name=AHFS2016/> In those with ] a low dose is recommended.<ref name=WHO2008/> Hydralazine is in the ] family of medications, so it is believed to work by causing the ].<ref name=AHFS2016/> | |||
| <!-- Society and culture --> | |||
| However, this only has a short term effect on blood pressure, as the system will reset to the previous, high blood pressure necessary to maintain pressure in the kidney necessary for natriuresis. The long term effect of antihypertensive drugs comes from their effects on the pressure natriuresis curve. | |||
| Hydralazine was discovered while scientists at ] were looking for a treatment for malaria.<ref name=Wermuth>{{cite book | vauthors = Wermuth CG | author-link = Camille Georges Wermuth|title=The Practice of Medicinal Chemistry|publisher=Academic Press|isbn=9780080568775|page=12|url=https://books.google.com/books?id=Qmt1_DQkCpEC&pg=PA12|language=en|url-status=live|archive-url=https://web.archive.org/web/20170226131556/https://books.google.com/books?id=Qmt1_DQkCpEC&pg=PA12|archive-date=26 February 2017|date=2 May 2011}}</ref> It was patented in 1949.<ref>{{cite book|title=Progress in Drug Research/Fortschritte der Arzneimittelforschung/Progrés des recherches pharmaceutiques|date=2013|publisher=Birkhäuser|isbn=9783034870948|page=206|url=https://books.google.com/books?id=i8LzBwAAQBAJ&pg=PA206|language=en|url-status=live|archive-url=https://web.archive.org/web/20161220130333/https://books.google.ca/books?id=i8LzBwAAQBAJ&pg=PA206|archive-date=20 December 2016}}</ref> It is on the ].<ref name="WHO23rd">{{cite book | vauthors = ((World Health Organization)) | title = The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023) | year = 2023 | hdl = 10665/371090 | author-link = World Health Organization | publisher = World Health Organization | location = Geneva | id = WHO/MHP/HPS/EML/2023.02 | hdl-access=free }}</ref> In 2022, it was the 121st most commonly prescribed medication in the United States, with more than 5{{nbsp}}million prescriptions.<ref>{{cite web | title=The Top 300 of 2022 | url=https://clincalc.com/DrugStats/Top300Drugs.aspx | website=ClinCalc | access-date=30 August 2024 | archive-date=30 August 2024 | archive-url=https://web.archive.org/web/20240830202410/https://clincalc.com/DrugStats/Top300Drugs.aspx | url-status=live }}</ref><ref>{{cite web | title = Hydralazine Drug Usage Statistics, United States, 2013 - 2022 | website = ClinCalc | url = https://clincalc.com/DrugStats/Drugs/Hydralazine | access-date = 30 August 2024 }}</ref> | |||
| == |
== Medical use == | ||
| Hydralazine is not used as a primary drug for treating hypertension because it elicits a reflex ] stimulation of the heart (the ]).<ref name=Cochrane2011rev>{{cite journal | vauthors = Kandler MR, Mah GT, Tejani AM, Stabler SN, Salzwedel DM | title = Hydralazine for essential hypertension | journal = The Cochrane Database of Systematic Reviews | issue = 11 | pages = CD004934 | date = November 2011 | pmid = 22071816 | doi = 10.1002/14651858.CD004934.pub4 }}</ref> The sympathetic stimulation may increase heart rate and ], and in people with coronary artery disease may cause ] or ].<ref name=Harvey/> Hydralazine may also increase ] ] concentration, resulting in fluid retention. To prevent these undesirable side effects, hydralazine is usually prescribed in combination with a ] (e.g., ]) and a ].<ref name=Harvey/> | |||
| Hydralazine increases ] levels, decreasing the action of the ] ], limiting ] from the ] of smooth muscle. This results in an vessel relaxation. It dilates arterioles more than veins.<ref name="First Aid">Le, Bhushan and Vasan. First Aid for the USMLE Step 1. 20th Anniv. Ed, 2010.</ref> | |||
| Hydralazine is used to treat severe hypertension, but is not a first-line therapy for ]. Hydralazine is often used to treat hypertension in pregnancy, though, with either labetalol and/or ].<ref name=Bhushan>{{cite book | vauthors = Bhushan V, Lee TT, Ozturk A | title = First Aid for the USMLE Step 1. | location = New York | publisher = McGraw-Hill Medical | date = 2007 | page = 251 }}</ref> | |||
| It recently has been identified as a ] donor.<ref name="urlantihtn">{{cite web |url=http://faculty.swosu.edu/scott.long/phcl/antihtn.htm |title=antihtn |work= |accessdate=2008-10-05}}</ref> | |||
| Hydralazine is commonly used in combination with ] for the treatment of ] in black populations. This preparation, ], was the first race-based prescription drug.<ref name=Ferdinand2014rev>{{cite journal | vauthors = Ferdinand KC, Elkayam U, Mancini D, Ofili E, Piña I, Anand I, Feldman AM, McNamara D, Leggett C | display-authors = 6 | title = Use of isosorbide dinitrate and hydralazine in African-Americans with heart failure 9 years after the African-American Heart Failure Trial | journal = The American Journal of Cardiology | volume = 114 | issue = 1 | pages = 151–9 | date = July 2014 | pmid = 24846808 | doi = 10.1016/j.amjcard.2014.04.018 | url = http://www.ajconline.org/article/S0002-9149(14)00975-8/pdf | doi-access = free }}</ref> | |||
| Activation of ] has been suggested as a mechanism.<ref name="pmid15192023">{{cite journal |author=Knowles HJ, Tian YM, Mole DR, Harris AL |title=Novel mechanism of action for hydralazine: induction of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases |journal=Circ. Res. |volume=95 |issue=2 |pages=162–9 |year=2004 |month=July |pmid=15192023 |doi=10.1161/01.RES.0000134924.89412.70 |url=http://circres.ahajournals.org/cgi/pmidlookup?view=long&pmid=15192023}}</ref> | |||
| It should not be used in people who have ], heart failure, constrictive ], ], a dissecting ], or ].<ref name=UKlabel2016/> | |||
| == Clinical Use == | |||
| Hydralazine is not used as a primary drug for treating hypertension because it elicits a reflex ] stimulation of the heart (the ]). The sympathetic stimulation may increase heart rate and ], and in patients with coronary artery disease may cause ] or ].<ref name=Harvey/> Hydralazine may also increase ] ] concentration, resulting in fluid retention. In order to prevent these undesirable side-effects, hydralazine is usually prescribed in combination with a beta-blocker (e.g., ]) and a ].<ref name=Harvey/> | |||
| == Adverse effects == | |||
| Hydralazine is used to treat severe hypertension, but again, it is not a first-line therapy for essential ]. However, hydralazine is the first-line therapy for hypertension in pregnancy, with ].<ref name=Bhushan>Bhushan, Vikas, Tao T. Lee, and Ali Ozturk. First Aid for the USMLE Step 1. New York: McGraw-Hill Medical, 2007. 251.</ref> | |||
| Prolonged treatment may cause a ], which can become fatal if the symptoms are not noticed and drug treatment stopped.<ref name =UKlabel2016/> Hydralazine is within the top three drugs that is known to induce systemic lupus and this adverse drug event is dose dependent yet significant. | |||
| Very common (>10% frequency) side effects include headache, tachycardia, and ].<ref name=UKlabel2016>{{cite web|title=Hydralazine Tablets 50mg|url=https://www.medicines.org.uk/emc/medicine/24412|work=UK Electronic Medicines Compendium|date=7 September 2016|language=en|url-status=live|archive-url=https://web.archive.org/web/20170227063940/https://www.medicines.org.uk/emc/medicine/24412|archive-date=27 February 2017}}</ref> | |||
| ===Pre-clinical research=== | |||
| Multiple sclerosis: Due to its ability to damage ] nerve sheaths, acrolein may be a factor in the development of ]. Hydralazine, a known scavenger of acrolein, was found to reduce myelin damage and significantly improve behavioral outcomes in a mouse model of multiple sclerosis (]).<ref>{{cite journal | last =Leung | first =G | coauthors =Sun W, Zheng L, Brookes S, Tully M, Shi R | title =Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune enchephalomyelitis mouse| journal = Neuroscience | volume =173 | pages =150| year =2010 | pmid =21081153 | doi=10.1016/j.neuroscience.2010.11.018 | pmc=3034379}}</ref> | |||
| Common (1–10% frequency) side effects include ], ], anginal symptoms, aching or swelling joints, muscle aches, positive tests for ], stomach upset, diarrhea, nausea and vomiting, and ] (sodium and water retention).<ref name =UKlabel2016/> | |||
| == Side effects == | |||
| Common side-effects include: | |||
| * ] | |||
| * Compensatory ] due to baroreceptor reflex ->Angina | |||
| * ] | |||
| * ] | |||
| * ] or ] | |||
| * ] | |||
| * ] | |||
| * ] deficiency <ref>http://query.nytimes.com/gst/fullpage.html?res=9807E7DF163FF935A35755C0A9669D8B63&n=Top/Features/Magazine/Columns/Diagnosis&pagewanted</ref> | |||
| * ]<ref>Schoonen WM,et al. Do selected drugs increase the risk of lupus? A matched case-control study. Br J Clin Pharmacol. 2010 Oct;70(4):588-96. doi: 10.1111/j.1365-2125.2010.03733.x. | |||
| </ref><ref name="pmid17404230">{{cite journal |author=Mazari L, Ouarzane M, Zouali M |title=Subversion of B lymphocyte tolerance by hydralazine, a potential mechanism for drug-induced lupus |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=104 |issue=15 |pages=6317–22 |year=2007 |month=April |pmid=17404230 |pmc=1851062 |doi=10.1073/pnas.0610434104 |url=http://www.pnas.org/cgi/pmidlookup?view=long&pmid=17404230}}</ref> | |||
| * ] - Generally MPO-ANCA positive | |||
| == Interactions == | |||
| Patients given hydralazine over a period of six months may develop a ]-like syndrome or other ]s that, in general, are reversible with withdrawal.<ref name=Harvey/> Hydralazine is differentially acetylated by fast and slow ] phenotypes, hence incidence of lupus-like disease in slow acetylators.{{Citation needed|date=May 2010}} | |||
| It may potentiate the antihypertensive effects of:<ref name =UKlabel2016/> | |||
| {{div col|colwidth=22em}} | |||
| * ] | |||
| * ]s | |||
| * ]s | |||
| * ]s | |||
| * ] | |||
| * ]s | |||
| * ] | |||
| * ] (]) | |||
| * ] | |||
| {{div col end}} | |||
| Drugs subject to a strong first-pass effect such as beta blockers may increase the ] of hydralazine.<ref name =UKlabel2016/> The heart rate-accelerating effects of ] (adrenaline) are increased by hydralazine, and coadministration may lead to toxicity.<ref name =UKlabel2016/> | |||
| == See also == | |||
| * ] | |||
| == Mechanism of action == | |||
| * ] | |||
| Hydralazine is a direct-acting ] relaxant and acts as a ] primarily in ], also known as the smooth muscle of the arterial bed. The molecular mechanism involves inhibition of inositol trisphosphate-induced Ca<sup>2+</sup> release from the sarcoplasmic reticulum in arterial smooth muscle cells.<ref name="BJP Allam">{{Cite journal| vauthors = Gurney AM, Allam M | title = Inhibition of calcium release from the sarcoplasmic reticulum of rabbit aorta by hydralazine | journal = British Journal of Pharmacology | volume = 114 | issue = 1 | pages = 238–244 | date = January 1995 | pmid = 7712024 | pmc = 1510175 | doi = 10.1111/j.1476-5381.1995.tb14931.x }}</ref><ref name="BJP Ellershaw">{{Cite journal| vauthors = Ellershaw DC, Gurney AM| title = Mechanisms of hydralazine induced vasodilation in rabbit aorta and pulmonary artery | journal = British Journal of Pharmacology | volume = 134 | issue = 3 | pages = 621–631 | date = October 2001 | pmid = 11588117 | pmc = 1572994 | doi = 10.1038/sj.bjp.0704302 }}</ref> By relaxing ], vasodilators act to decrease ], thereby lowering ] and decreasing afterload.<ref name=Harvey>{{cite book | vauthors = Harvey RA, Harvey PA, Mycek MJ | title = Lippincott's Illustrated Reviews: Pharmacology. | edition = 2nd | location = Philadelphia | publisher = Lippincott Williams & Wilkins | date = 2000 | page = 190 }}</ref> The exact ] of hydralazine is unknown, at least as of 1981.<ref name="Reece1981">{{cite journal | vauthors = Reece PA | title = Hydralazine and related compounds: chemistry, metabolism, and mode of action | journal = Med Res Rev | volume = 1 | issue = 1 | pages = 73–96 | date = 1981 | pmid = 7050561 | doi = 10.1002/med.2610010105 | url = }}</ref> | |||
| * ] | |||
| Metabolic products include the ''N''-] derivative, ], and ], each of which may also contribute to reducing blood pressure.<ref>{{cite journal |title= Direct-acting vasodilators |vauthors= Cohn JN, McInnes GT, Shepherd AM |journal= Journal of Clinical Hypertension |year= 2011 |volume= 13 |issue= 9 |pages= 690–692 |doi= 10.1111/j.1751-7176.2011.00507.x |pmid= 21896152 |pmc= 8108999 }}</ref> | |||
| ==Chemistry== | |||
| Hydralazine belongs to the ] class of drugs.<ref name=Schroeder>{{cite journal | vauthors = Schroeder NA | title = The effect of 1-hydrasinophthalasine in hypertension | journal = Circulation | volume = 5 | issue = 1 | pages = 28–37 | date = January 1952 | pmid = 14896450 | doi = 10.1161/01.cir.5.1.28 | doi-access = free }}</ref> | |||
| ==History== | |||
| The antihypertensive activity of hydralazine was discovered by scientists at Ciba, who were trying to discover drugs to treat malaria; it was initially called C-5968 and 1-hydrazinophthalazine; Ciba's patent application was filed in 1945 and issued in 1949,<ref>{{cite web|title=Hydralazine|url=https://www.drugbank.ca/drugs/DB01275|publisher=Drugbank|access-date=4 March 2017|url-status=live|archive-url=https://web.archive.org/web/20170304114322/https://www.drugbank.ca/drugs/DB01275|archive-date=4 March 2017}}</ref><ref>{{cite web|title=hydralazine|url=https://pubchem.ncbi.nlm.nih.gov/compound/hydralazine#section=Physical-Description|publisher=PubChem|access-date=4 March 2017|language=en|url-status=live|archive-url=https://web.archive.org/web/20170304115216/https://pubchem.ncbi.nlm.nih.gov/compound/hydralazine#section=Physical-Description|archive-date=4 March 2017}}</ref><ref>; see Example 1</ref> and the first scientific publications of its blood pressure-lowering activities appeared in 1950.<ref name=Wermuth/><ref name=Schroeder/><ref>{{cite journal | vauthors = Reubi FC | title = Renal hyperemia induced in man by a new phthalazine derivative | journal = Proceedings of the Society for Experimental Biology and Medicine | volume = 73 | issue = 1 | pages = 102–103 | date = January 1950 | pmid = 15402536 | doi = 10.3181/00379727-73-17591 | s2cid = 32603042 }}</ref> It was approved by the FDA in 1953.<ref>{{cite web|title=New Drug Application (NDA) 008303 Company: NOVARTIS Drug Name(s): Apresoline|url=http://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=008303|publisher=FDA|access-date=26 February 2017|url-status=live|archive-url=https://web.archive.org/web/20170226135437/http://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=008303|archive-date=26 February 2017}}</ref> | |||
| It was one of the first antihypertensive medications that could be taken by mouth.<ref name=Cochrane2011rev/> | |||
| ==Research== | |||
| Hydralazine has also been studied as a treatment for ] in its capacity as a ] inhibitor.<ref>{{cite journal | vauthors = Singh V, Sharma P, Capalash N | title = DNA methyltransferase-1 inhibitors as epigenetic therapy for cancer | journal = Current Cancer Drug Targets | volume = 13 | issue = 4 | pages = 379–99 | date = May 2013 | pmid = 23517596 | doi = 10.2174/15680096113139990077 }}</ref> | |||
| == References == | == References == | ||
| {{Reflist |
{{Reflist}} | ||
| {{Nonsympatholytic vasodilatory antihypertensives}} | {{Nonsympatholytic vasodilatory antihypertensives}} | ||
| {{Monoamine metabolism modulators}} | |||
| {{Hydrazines}} | {{Hydrazines}} | ||
| {{Portal bar|Medicine}} | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| {{antihypertensive-stub}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 03:59, 30 September 2024
Anti-hypertension medication Not to be confused with hydrazine.Pharmaceutical compound
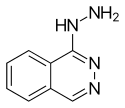 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Apresoline, BiDil, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682246 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 26–50% |
| Protein binding | 85–90% |
| Metabolism | Liver |
| Onset of action | 5 to 30 min |
| Elimination half-life | 2–8 hours, 7–16 hours (renal impairment) |
| Duration of action | 2 to 6 hrs |
| Excretion | Urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.528 |
| Chemical and physical data | |
| Formula | C8H8N4 |
| Molar mass | 160.180 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Hydralazine, sold under the brand name Apresoline among others, is a medication used to treat high blood pressure and heart failure. This includes high blood pressure in pregnancy and very high blood pressure resulting in symptoms. It has been found to be particularly useful in heart failure, together with isosorbide dinitrate, for treatment of people of African descent. It is given by mouth or by injection into a vein. Effects usually begin around 15 minutes and last up to six hours.
Common side effects include headache and fast heart rate. It is not recommended in people with coronary artery disease or in those with rheumatic heart disease that affects the mitral valve. In those with kidney disease a low dose is recommended. Hydralazine is in the vasodilator family of medications, so it is believed to work by causing the dilation of blood vessels.
Hydralazine was discovered while scientists at Ciba were looking for a treatment for malaria. It was patented in 1949. It is on the World Health Organization's List of Essential Medicines. In 2022, it was the 121st most commonly prescribed medication in the United States, with more than 5 million prescriptions.
Medical use
Hydralazine is not used as a primary drug for treating hypertension because it elicits a reflex sympathetic stimulation of the heart (the baroreceptor reflex). The sympathetic stimulation may increase heart rate and cardiac output, and in people with coronary artery disease may cause angina pectoris or myocardial infarction. Hydralazine may also increase plasma renin concentration, resulting in fluid retention. To prevent these undesirable side effects, hydralazine is usually prescribed in combination with a beta blocker (e.g., propranolol) and a diuretic.
Hydralazine is used to treat severe hypertension, but is not a first-line therapy for essential hypertension. Hydralazine is often used to treat hypertension in pregnancy, though, with either labetalol and/or methyldopa.
Hydralazine is commonly used in combination with isosorbide dinitrate for the treatment of congestive heart failure in black populations. This preparation, isosorbide dinitrate/hydralazine, was the first race-based prescription drug.
It should not be used in people who have tachycardia, heart failure, constrictive pericarditis, lupus, a dissecting aortic aneurysm, or porphyria.
Adverse effects
Prolonged treatment may cause a syndrome similar to lupus, which can become fatal if the symptoms are not noticed and drug treatment stopped. Hydralazine is within the top three drugs that is known to induce systemic lupus and this adverse drug event is dose dependent yet significant.
Very common (>10% frequency) side effects include headache, tachycardia, and palpitations.
Common (1–10% frequency) side effects include flushing, hypotension, anginal symptoms, aching or swelling joints, muscle aches, positive tests for atrial natriuretic peptide, stomach upset, diarrhea, nausea and vomiting, and swelling (sodium and water retention).
Interactions
It may potentiate the antihypertensive effects of:
- Vasodilators
- Calcium antagonists
- ACE inhibitors
- Diuretics
- Antihypertensives
- Tricyclic antidepressants
- Major tranquillisers
- Ethanol (alcohol)
- Diazoxide
Drugs subject to a strong first-pass effect such as beta blockers may increase the bioavailability of hydralazine. The heart rate-accelerating effects of epinephrine (adrenaline) are increased by hydralazine, and coadministration may lead to toxicity.
Mechanism of action
Hydralazine is a direct-acting smooth muscle relaxant and acts as a vasodilator primarily in resistance arterioles, also known as the smooth muscle of the arterial bed. The molecular mechanism involves inhibition of inositol trisphosphate-induced Ca release from the sarcoplasmic reticulum in arterial smooth muscle cells. By relaxing vascular smooth muscle, vasodilators act to decrease peripheral resistance, thereby lowering blood pressure and decreasing afterload. The exact mechanism of action of hydralazine is unknown, at least as of 1981.
Metabolic products include the N-acetyl derivative, pyruvic acid hydrazone, and acetone hydrazone, each of which may also contribute to reducing blood pressure.
Chemistry
Hydralazine belongs to the hydrazinophthalazine class of drugs.
History
The antihypertensive activity of hydralazine was discovered by scientists at Ciba, who were trying to discover drugs to treat malaria; it was initially called C-5968 and 1-hydrazinophthalazine; Ciba's patent application was filed in 1945 and issued in 1949, and the first scientific publications of its blood pressure-lowering activities appeared in 1950. It was approved by the FDA in 1953.
It was one of the first antihypertensive medications that could be taken by mouth.
Research
Hydralazine has also been studied as a treatment for myelodysplastic syndrome in its capacity as a DNA methyltransferase inhibitor.
References
- "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 30 March 2024.
- ^ "Hydralazine Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 280. hdl:10665/44053. ISBN 9789241547659.
- ^ Wermuth CG (2 May 2011). The Practice of Medicinal Chemistry. Academic Press. p. 12. ISBN 9780080568775. Archived from the original on 26 February 2017.
- Progress in Drug Research/Fortschritte der Arzneimittelforschung/Progrés des recherches pharmaceutiques. Birkhäuser. 2013. p. 206. ISBN 9783034870948. Archived from the original on 20 December 2016.
- World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- "Hydralazine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ Kandler MR, Mah GT, Tejani AM, Stabler SN, Salzwedel DM (November 2011). "Hydralazine for essential hypertension". The Cochrane Database of Systematic Reviews (11): CD004934. doi:10.1002/14651858.CD004934.pub4. PMID 22071816.
- ^ Harvey RA, Harvey PA, Mycek MJ (2000). Lippincott's Illustrated Reviews: Pharmacology (2nd ed.). Philadelphia: Lippincott Williams & Wilkins. p. 190.
- Bhushan V, Lee TT, Ozturk A (2007). First Aid for the USMLE Step 1. New York: McGraw-Hill Medical. p. 251.
- Ferdinand KC, Elkayam U, Mancini D, Ofili E, Piña I, Anand I, et al. (July 2014). "Use of isosorbide dinitrate and hydralazine in African-Americans with heart failure 9 years after the African-American Heart Failure Trial". The American Journal of Cardiology. 114 (1): 151–9. doi:10.1016/j.amjcard.2014.04.018. PMID 24846808.
- ^ "Hydralazine Tablets 50mg". UK Electronic Medicines Compendium. 7 September 2016. Archived from the original on 27 February 2017.
- Gurney AM, Allam M (January 1995). "Inhibition of calcium release from the sarcoplasmic reticulum of rabbit aorta by hydralazine". British Journal of Pharmacology. 114 (1): 238–244. doi:10.1111/j.1476-5381.1995.tb14931.x. PMC 1510175. PMID 7712024.
- Ellershaw DC, Gurney AM (October 2001). "Mechanisms of hydralazine induced vasodilation in rabbit aorta and pulmonary artery". British Journal of Pharmacology. 134 (3): 621–631. doi:10.1038/sj.bjp.0704302. PMC 1572994. PMID 11588117.
- Reece PA (1981). "Hydralazine and related compounds: chemistry, metabolism, and mode of action". Med Res Rev. 1 (1): 73–96. doi:10.1002/med.2610010105. PMID 7050561.
- Cohn JN, McInnes GT, Shepherd AM (2011). "Direct-acting vasodilators". Journal of Clinical Hypertension. 13 (9): 690–692. doi:10.1111/j.1751-7176.2011.00507.x. PMC 8108999. PMID 21896152.
- ^ Schroeder NA (January 1952). "The effect of 1-hydrasinophthalasine in hypertension". Circulation. 5 (1): 28–37. doi:10.1161/01.cir.5.1.28. PMID 14896450.
- "Hydralazine". Drugbank. Archived from the original on 4 March 2017. Retrieved 4 March 2017.
- "hydralazine". PubChem. Archived from the original on 4 March 2017. Retrieved 4 March 2017.
- US2484029; see Example 1
- Reubi FC (January 1950). "Renal hyperemia induced in man by a new phthalazine derivative". Proceedings of the Society for Experimental Biology and Medicine. 73 (1): 102–103. doi:10.3181/00379727-73-17591. PMID 15402536. S2CID 32603042.
- "New Drug Application (NDA) 008303 Company: NOVARTIS Drug Name(s): Apresoline". FDA. Archived from the original on 26 February 2017. Retrieved 26 February 2017.
- Singh V, Sharma P, Capalash N (May 2013). "DNA methyltransferase-1 inhibitors as epigenetic therapy for cancer". Current Cancer Drug Targets. 13 (4): 379–99. doi:10.2174/15680096113139990077. PMID 23517596.
| Nonsympatholytic vasodilatory antihypertensives (C02) | |
|---|---|
| Nitrovasodilator (arterioles and venules) | |
| Hydrazinophthalazines (arterioles) | |
| Potassium channel openers (arterioles) | |
| Calcium channel blockers (arterioles) |
|
| |
| Hydrazines | |
|---|---|
|