| Revision as of 22:04, 10 January 2012 edit60.239.85.102 (talk) →Legal status← Previous edit | Latest revision as of 22:46, 23 November 2024 edit undoReba16 (talk | contribs)Extended confirmed users8,067 editsNo edit summary | ||
| (113 intermediate revisions by 62 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{Drugbox | {{Drugbox | ||
| | Verifiedfields = changed | |||
| | verifiedrevid = 470614581 | |||
| | IUPAC_name = 1-piperazine | | IUPAC_name = 1-piperazine | ||
| | image = TFMPP.svg | | image = TFMPP.svg | ||
| Line 10: | Line 13: | ||
| | pregnancy_category = | | pregnancy_category = | ||
| | legal_AU = S9 | | legal_AU = S9 | ||
| | legal_BR = F2 | |||
| ⚫ | | legal_status = |
||
| | legal_BR_comment = <ref>{{Cite web |author=Anvisa |author-link=Brazilian Health Regulatory Agency |date=2023-07-24 |title=RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial |trans-title=Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control|url=https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-804-de-24-de-julho-de-2023-498447451 |url-status=live |archive-url=https://web.archive.org/web/20230827163149/https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-804-de-24-de-julho-de-2023-498447451 |archive-date=2023-08-27 |access-date=2023-08-27 |publisher=] |language=pt-BR |publication-date=2023-07-25}}</ref> | |||
| | legal_CA = Schedule III | |||
| | legal_DE = Anlage II | |||
| | legal_US = Scheduled in Florida; Unscheduled Federally | |||
| | legal_NZ = Class C | |||
| ⚫ | | legal_status = II-P (Poland)<ref>{{cite web | title = Ustawa z dnia 15 kwietnia 2011 r. o zmianie ustawy o przeciwdziałaniu narkomanii ( Dz.U. 2011 nr 105 poz. 614 ) | url = http://isap.sejm.gov.pl/DetailsServlet?id=WDU20111050614 | publisher = Internetowy System Aktów Prawnych | access-date = 17 June 2011}}</ref> | ||
| | routes_of_administration = Oral | | routes_of_administration = Oral | ||
| Line 16: | Line 25: | ||
| | bioavailability = | | bioavailability = | ||
| | protein_bound = | | protein_bound = | ||
| | metabolism = | | metabolism = ]<br />], ], ] | ||
| | elimination_half-life = | | elimination_half-life = | ||
| | excretion = |
| excretion = | ||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | CAS_number_Ref = {{cascite|changed|??}} | |||
| | CAS_number = 15532-75-9 | | CAS_number = 15532-75-9 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 25R3ONU51C | |||
| | ATC_prefix = none | | ATC_prefix = none | ||
| | ATC_suffix = | | ATC_suffix = | ||
| | PubChem = 4296 | | PubChem = 4296 | ||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | DrugBank = | | DrugBank = | ||
| | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| | ChemSpiderID = 4145 | | ChemSpiderID = 4145 | ||
| | ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| | ChEBI = 83536 | |||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | KEGG = C22745 | |||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | C=11 | H=13 | F=3 | N=2 | | C=11 | H=13 | F=3 | N=2 | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | molecular_weight = 230.23 g/mol | |||
| | StdInChI = 1S/C11H13F3N2/c12-11(13,14)9-3-1-2-4-10(9)16-7-5-15-6-8-16/h1-4,15H,5-8H2 | | StdInChI = 1S/C11H13F3N2/c12-11(13,14)9-3-1-2-4-10(9)16-7-5-15-6-8-16/h1-4,15H,5-8H2 | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey = VZUBMIDXJRGARE-UHFFFAOYSA-N | | StdInChIKey = VZUBMIDXJRGARE-UHFFFAOYSA-N | ||
| }} | }} | ||
| ] | |||
| ⚫ | ] | ||
| '''3-Trifluoromethylphenylpiperazine''' ('''TFMPP''') is a ] of the ] ]. Usually in combination with |
'''3-Trifluoromethylphenylpiperazine''' ('''TFMPP''') is a ] of the ] ] and is a ]. Usually in combination with ] (BZP) and other ]s, it is sold as an alternative to the illicit drug ] ("Ecstasy").<ref>{{cite web |url=https://www.erowid.org/chemicals/tfmpp/tfmpp_basics.shtml |title=Erowid TFMPP Vault: Basics |publisher=Erowid |access-date=2014-02-15}}</ref><ref name="Schep2011">{{cite journal |vauthors=Schep LJ, Slaughter RJ, Vale JA, Beasley DM, Gee P |title=The clinical toxicology of the designer "party pills" benzylpiperazine and trifluoromethylphenylpiperazine |journal=] |volume=49 |issue=3 |pages=131–41 |date=March 2011 |pmid=21495881 |doi=10.3109/15563650.2011.572076 |s2cid=42491343 }}</ref> | ||
| == Pharmacology == | == Pharmacology == | ||
| ⚫ | TFMPP has ] for the ] (K<sub>i</sub> = 288 nM), ] (K<sub>i</sub> = 132 nM), ] (K<sub>i</sub> = 282 nM), ] (K<sub>i</sub> = 269 nM), and ] (K<sub>i</sub> = 62 nM) ]s, and functions as a ] at all sites except the 5-HT<sub>2A</sub> receptor, where it acts as a weak ] or ].<ref name="pmid15496938">{{cite journal |vauthors=Baumann MH, Clark RD, Budzynski AG, Partilla JS, Blough BE, Rothman RB | title = N-substituted piperazines abused by humans mimic the molecular mechanism of 3,4-methylenedioxymethamphetamine (MDMA, or 'Ecstasy') | journal = Neuropsychopharmacology | volume = 30 | issue = 3 | pages = 550–60 |date=March 2005 | pmid = 15496938 | doi = 10.1038/sj.npp.1300585 | s2cid = 24217984 | doi-access = free }}</ref> Unlike the related piperazine compound ] (mCPP), TFMPP has insignificant affinity for the ] (IC<sub>50</sub> = 2,373 nM).<ref name="pmid1736030">{{cite journal |vauthors=Robertson DW, Bloomquist W, Wong DT, Cohen ML | title = mCPP but not TFMPP is an antagonist at cardiac 5HT3 receptors | journal = Life Sciences | volume = 50 | issue = 8 | pages = 599–605 | year = 1992 | pmid = 1736030 | doi = 10.1016/0024-3205(92)90372-V}}</ref> TFMPP also binds to the ] (EC<sub>50</sub> = 121 nM) and evokes the ] of ].<ref name="pmid15496938"/> It has no effects on ] or ] ] or efflux.<ref name="pmid15496938"/> | ||
| ⚫ | TFMPP has ] for the ] (K<sub>i</sub> = 288 |
||
| == Use and effects == | == Use and effects == | ||
| ⚫ | TFMPP is rarely used by itself. In fact, TFMPP ] and produces ] effects in animals rather than self-administration, which may explain the decision of the DEA not to permanently make TFMPP a controlled substance.<ref name="pmid15496938"/> More commonly, TFMPP is co-administered with BZP, which acts as a norepinephrine and dopamine releasing agent.<ref>{{cite journal |vauthors=Partilla JS, Dempsey AG, Nagpal AS, Blough BE, Baumann MH, Rothman RB |title=Interaction of amphetamines and related compounds at the vesicular monoamine transporter |journal=Journal of Pharmacology and Experimental Therapeutics |date=October 2006 |volume=319 |issue=1 |pages=237–46 |doi=10.1124/jpet.106.103622 |pmid=16835371|citeseerx=10.1.1.690.6669 |s2cid=22730478 }}</ref> Due to the serotonin agonist effects and increase in serotonin, norepinephrine, and dopamine levels produced by the BZP/TFMPP combination, this mixture of drugs produces effects which crudely mimic those of ].<ref>{{cite journal |vauthors=Yarosh HL, Katz EB, Coop A, Fantegrossi WE |title=MDMA-like behavioral effects of N-substituted piperazines in the mouse |journal=Pharmacology Biochemistry and Behavior |date=November 2007 |volume=88 |issue=1 |pages=18–27 |doi=10.1016/j.pbb.2007.06.007|pmid=17651790 |pmc=2082056 }}</ref> | ||
| == Side effects == | |||
| ⚫ | TFMPP is rarely used by itself. In fact, TFMPP reduces locomotor activity and produces aversive effects in animals rather than self-administration, which |
||
| ⚫ | The combination of BZP and TFMPP has been associated with a range of side effects, including ], ], ] and vomiting, ] and muscle aches which may resemble ], ], ], and rarely ],<ref name="Schep2011"/> as well as a prolonged and unpleasant ] effect. These side effects tend to be significantly worsened when the BZP/TFMPP mix is consumed alongside ], especially the headache, nausea, and hangover. | ||
| ⚫ | However, it is difficult to say how many of these side effects are produced by TFMPP itself, as it has rarely been marketed without BZP also being present, and all of the side effects mentioned are also produced by BZP (which has been sold as a single drug). Studies into other related piperazine drugs such as ] suggest that certain side effects such as ], ] and ] are common to all drugs of this class, and pills containing TFMPP are reported by users to produce comparatively more severe hangover effects than those containing only BZP. The drug can also cause the body to tremble for a long period of time.<ref>{{cite web | vauthors =Wilkins C, Girling M, Sweetsur P, Huckle T, Huakau J | title =Legal party pill use in New Zealand: Prevalence of use, availability, health harms and 'gateway effects' of benzylpiperazine (BZP) and trifluorophenylmethylpiperazine (TFMPP) | publisher =Centre for Social and Health Outcomes Research and Evaluation (SHORE) | url =http://www.spiritualhigh.co.uk/spiritualhigh.co.uk/downloads/Legal-party-pills-in-New-Zealand-report.pdf | access-date =2007-04-14 | url-status =dead | archive-url =https://web.archive.org/web/20070318042503/http://www.spiritualhigh.co.uk/spiritualhigh.co.uk/downloads/Legal-party-pills-in-New-Zealand-report.pdf | archive-date =2007-03-18 }}</ref>{{unreliable source?|date=February 2015}} | ||
| The dosage commonly used when combined with BZP for "ecstasy-like effects" is between 30 and 100 mg, while higher doses of TFMPP alone cause mildly hallucinogenic effects at around 100–250 mg; however, higher doses can cause a range of side effects including ] headaches, muscle aches, ] and vomiting, as well as a come-down syndrome characterized by ], ], and ] (see below). These side effects tend to discourage use of TFMPP. | |||
| == |
== Legal status == | ||
| ===Canada=== | |||
| ⚫ | The combination of BZP and TFMPP has been associated with a range of side effects, including ], ], ] and vomiting, ] and muscle aches which may resemble ], ], ], and rarely ],<ref name="Schep2011"/> as well as a prolonged and unpleasant hangover effect |
||
| Since 2012, TFMPP has been listed as a Schedule III controlled substance in ],<ref>{{cite web|title=SOR/2012-65 March 30, 2012 Controlled Drugs and Substances Act|url=http://gazette.gc.ca/rp-pr/p2/2012/2012-04-11/html/sor-dors65-eng.html#archived|website=Canada Gazette|publisher=Government of Canada|access-date=15 September 2014}}</ref> making possession of TFMPP a federal offence. It has also been added to Part J of the Food and Drug Regulations thereby prohibiting the production, export or import of the substance. | |||
| ===China=== | |||
| ⚫ | However, it is difficult to say how many of these side effects are produced by TFMPP itself, as it has rarely been marketed without BZP also being present, and all of the side effects mentioned are also produced by BZP (which has been sold as a single drug). Studies into other related piperazine drugs such as ] suggest that certain side effects such as ], ] and ] are common to all drugs of this class, and pills containing TFMPP are reported by users to produce comparatively more severe hangover effects than those containing only BZP. The drug can also cause the body to tremble for a long period of time.<ref>{{cite web | |
||
| As of October 2015 TFMPP is a controlled substance in China.<ref>{{cite web | url=http://www.sfda.gov.cn/WS01/CL0056/130753.html | title=关于印发《非药用类麻醉药品和精神药品列管办法》的通知 | publisher=China Food and Drug Administration | date=27 September 2015 | language=zh | access-date=1 October 2015}}</ref> | |||
| == Legal status == | |||
| ===Finland=== | |||
| ⚫ | ] | ||
| Scheduled in government decree on psychoactive substances banned from the consumer market.<ref>{{cite web | url=https://www.finlex.fi/fi/laki/alkup/2015/20151129 | title=FINLEX ® - Säädökset alkuperäisinä: Valtioneuvoston asetus kuluttajamarkkinoilta… 1129/2015 }}</ref><ref>{{cite web | url=https://www.finlex.fi/fi/laki/alkup/2017/20170225 | title=FINLEX ® - Säädökset alkuperäisinä: Valtioneuvoston asetus kuluttajamarkkinoilta… 225/2017 }}</ref><ref>{{cite web | url=https://www.finlex.fi/fi/laki/alkup/2021/20210733 | title=FINLEX ® - Säädökset alkuperäisinä: Valtioneuvoston asetus kuluttajamarkkinoilta… 733/2021 }}</ref> | |||
| ===Denmark=== | |||
| As of December 3, 2005, TFMPP is illegal in Denmark. As of March 1, 2006, TFMPP is scheduled as a "dangerous substance" in Sweden.<ref></ref> TFMPP is unscheduled in the ]. TFMPP was briefly emergency scheduled in ] in the USA, but the scheduling expired in April 2004 and has not been renewed.<ref>U.S. Department of Justice, Drug Enforcement Administration (DEA), </ref> Therefore, unlike its cousin ], TFMPP is not currently an illicit drug under United States federal law. However, some states have banned the drug in their criminal statutes making its possession a felony. | |||
| As of December 3, 2005, TFMPP is illegal in ]. | |||
| ===Japan=== | |||
| ⚫ | Based on the recommendation of the EACD, the New Zealand government has passed legislation which placed BZP, along with the other piperazine derivatives TFMPP, mCPP, pFPP, MeOPP and MBZP, into Class C of the New Zealand Misuse of Drugs Act 1975. A ban was intended to come into effect in New Zealand on December 18, 2007, but the law change did not go through until the following year, and the sale of BZP and the other listed piperazines became illegal in New Zealand as of 1 April 2008. An amnesty for possession and usage of these drugs remained until October 2008, at which point they became completely illegal.<ref> |
||
| ⚫ | Since 2003, TFMPP and BZP became illegal in ]. | ||
| ===Netherlands=== | |||
| ⚫ | As of December 2009 TFMPP has been made a Class C drug in the |
||
| TFMPP is unscheduled in the ]. {{Citation needed|date=April 2023}} | |||
| ⚫ | Since 2003 TFMPP and BZP became illegal in Japan. | ||
| == |
===New Zealand=== | ||
| ⚫ | Based on the recommendation of the EACD, the ] government has passed legislation which placed BZP, along with the other piperazine derivatives TFMPP, mCPP, pFPP, MeOPP and MBZP, into Class C of the New Zealand Misuse of Drugs Act 1975. A ban was intended to come into effect in New Zealand on December 18, 2007, but the law change did not go through until the following year, and the sale of BZP and the other listed piperazines became illegal in New Zealand as of 1 April 2008. An amnesty for possession and usage of these drugs remained until October 2008, at which point they became completely illegal.<ref>{{cite web |url=http://www.parliament.nz/en-NZ/PB/Legislation/Bills/d/3/d/00DBHOH_BILL8220_1-Misuse-of-Drugs-Classification-of-BZP-Amendment.htm |title=Misuse of Drugs (Classification of BZP) Amendment Bill 2008}}</ref> | ||
| * ] (pTFMPP) | |||
| * ] (BZP) | |||
| ===Sweden=== | |||
| * ] (MBZP) | |||
| As of March 1, 2006, TFMPP is scheduled as a "dangerous substance" in ].<ref>{{cite web |url=https://www.erowid.org/chemicals/tfmpp/tfmpp_law.shtml |title=Erowid TFMPP Vault : Legal Status |publisher=Erowid}}</ref> | |||
| * ] (DBZP) | |||
| * ] (mCPP) | |||
| ===Switzerland=== | |||
| * ] (MDBZP) | |||
| As of December 1, 2010, TFMPP is a controlled substance in ].<ref>{{cite web |title=RO 2010 4099 |url=https://fedlex.data.admin.ch/eli/oc/2010/599 |publisher=Fedlex |access-date=2022-08-16}}</ref><ref>{{cite web | url=https://www.fedlex.admin.ch/eli/cc/2011/363/fr| title=RO 2011 2595 |publisher=Fedlex |access-date=2022-08-16}}</ref> | |||
| * ] (2C-B-BZP) | |||
| * ] (pFPP) | |||
| ===United Kingdom=== | |||
| * ] (MeOPP) | |||
| ⚫ | As of December 2009, TFMPP has been made a Class C drug in the ] along with BZP. | ||
| * ] | |||
| ===United States=== | |||
| TFMPP is not currently scheduled at the federal level in the ],<ref name="PART 1308 — SCHEDULES OF CONTROLLED SUBSTANCES - 1308.11 Schedule I">{{Cite web |url=http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm |title=§1308.11 Schedule I. |access-date=2014-12-17 |archive-date=2009-08-27 |archive-url=https://web.archive.org/web/20090827043725/http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm |url-status=dead }}</ref> but it was briefly emergency scheduled in ]. The scheduling expired in April 2004 and was not renewed.<ref>{{cite web |url=http://www.deadiversion.usdoj.gov/fed_regs/sched_actions/2002/fr09202.htm |title=Scheduling Actions 2002 |publisher=U.S. Department of Justice, Drug Enforcement Administration (DEA) |url-status=dead |archive-url=https://web.archive.org/web/20030102043925/http://www.deadiversion.usdoj.gov/fed_regs/sched_actions/2002/fr09202.htm |archive-date=2003-01-02 }}</ref> However, some states such as Florida have banned the drug in their criminal statutes making its possession a felony.<ref name="Florida Statutes - Chapter 893 - DRUG ABUSE PREVENTION AND CONTROL"></ref> | |||
| ====Florida==== | |||
| TFMPP is a Schedule I ] in the state of ] making it illegal to buy, sell, or possess in Florida.<ref name="Florida Statutes - Chapter 893 - DRUG ABUSE PREVENTION AND CONTROL"/> | |||
| ====Texas==== | |||
| TFMPP is controlled in ] under Penalty Group 2, as a hallucinogenic substance. It is illegal to possess TFMPP in any quantity in Texas. | |||
| ==Derivatives== | |||
| *] | |||
| *] | |||
| == References == | == References == | ||
| Line 80: | Line 118: | ||
| == External links == | == External links == | ||
| * | * | ||
| {{Entactogens}} | {{Entactogens}} | ||
| {{Serotonin receptor modulators}} | |||
| {{Serotonergics}} | |||
| {{Monoamine releasing agents}} | |||
| {{Piperazines}} | {{Piperazines}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 22:46, 23 November 2024
Chemical compound Pharmaceutical compound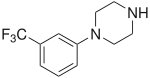 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver CYP2D6, CYP1A2, CYP3A4 |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.035.962 |
| Chemical and physical data | |
| Formula | C11H13F3N2 |
| Molar mass | 230.234 g·mol |
InChI
| |
| (what is this?) (verify) | |

3-Trifluoromethylphenylpiperazine (TFMPP) is a recreational drug of the phenylpiperazine chemical class and is a substituted piperazine. Usually in combination with benzylpiperazine (BZP) and other analogues, it is sold as an alternative to the illicit drug MDMA ("Ecstasy").
Pharmacology
TFMPP has affinity for the 5-HT1A (Ki = 288 nM), 5-HT1B (Ki = 132 nM), 5-HT1D (Ki = 282 nM), 5-HT2A (Ki = 269 nM), and 5-HT2C (Ki = 62 nM) receptors, and functions as a full agonist at all sites except the 5-HT2A receptor, where it acts as a weak partial agonist or antagonist. Unlike the related piperazine compound meta-chlorophenylpiperazine (mCPP), TFMPP has insignificant affinity for the 5-HT3 receptor (IC50 = 2,373 nM). TFMPP also binds to the SERT (EC50 = 121 nM) and evokes the release of serotonin. It has no effects on dopamine or norepinephrine reuptake or efflux.
Use and effects
TFMPP is rarely used by itself. In fact, TFMPP reduces locomotor activity and produces aversive effects in animals rather than self-administration, which may explain the decision of the DEA not to permanently make TFMPP a controlled substance. More commonly, TFMPP is co-administered with BZP, which acts as a norepinephrine and dopamine releasing agent. Due to the serotonin agonist effects and increase in serotonin, norepinephrine, and dopamine levels produced by the BZP/TFMPP combination, this mixture of drugs produces effects which crudely mimic those of MDMA.
Side effects
The combination of BZP and TFMPP has been associated with a range of side effects, including insomnia, anxiety, nausea and vomiting, headaches and muscle aches which may resemble migraine, seizures, impotence, and rarely psychosis, as well as a prolonged and unpleasant hangover effect. These side effects tend to be significantly worsened when the BZP/TFMPP mix is consumed alongside alcohol, especially the headache, nausea, and hangover.
However, it is difficult to say how many of these side effects are produced by TFMPP itself, as it has rarely been marketed without BZP also being present, and all of the side effects mentioned are also produced by BZP (which has been sold as a single drug). Studies into other related piperazine drugs such as mCPP suggest that certain side effects such as anxiety, headache and nausea are common to all drugs of this class, and pills containing TFMPP are reported by users to produce comparatively more severe hangover effects than those containing only BZP. The drug can also cause the body to tremble for a long period of time.
Legal status
Canada
Since 2012, TFMPP has been listed as a Schedule III controlled substance in Canada, making possession of TFMPP a federal offence. It has also been added to Part J of the Food and Drug Regulations thereby prohibiting the production, export or import of the substance.
China
As of October 2015 TFMPP is a controlled substance in China.
Finland
Scheduled in government decree on psychoactive substances banned from the consumer market.
Denmark
As of December 3, 2005, TFMPP is illegal in Denmark.
Japan
Since 2003, TFMPP and BZP became illegal in Japan.
Netherlands
TFMPP is unscheduled in the Netherlands.
New Zealand
Based on the recommendation of the EACD, the New Zealand government has passed legislation which placed BZP, along with the other piperazine derivatives TFMPP, mCPP, pFPP, MeOPP and MBZP, into Class C of the New Zealand Misuse of Drugs Act 1975. A ban was intended to come into effect in New Zealand on December 18, 2007, but the law change did not go through until the following year, and the sale of BZP and the other listed piperazines became illegal in New Zealand as of 1 April 2008. An amnesty for possession and usage of these drugs remained until October 2008, at which point they became completely illegal.
Sweden
As of March 1, 2006, TFMPP is scheduled as a "dangerous substance" in Sweden.
Switzerland
As of December 1, 2010, TFMPP is a controlled substance in Switzerland.
United Kingdom
As of December 2009, TFMPP has been made a Class C drug in the United Kingdom along with BZP.
United States
TFMPP is not currently scheduled at the federal level in the United States, but it was briefly emergency scheduled in Schedule I. The scheduling expired in April 2004 and was not renewed. However, some states such as Florida have banned the drug in their criminal statutes making its possession a felony.
Florida
TFMPP is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess in Florida.
Texas
TFMPP is controlled in Texas under Penalty Group 2, as a hallucinogenic substance. It is illegal to possess TFMPP in any quantity in Texas.
Derivatives
References
- Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- "Ustawa z dnia 15 kwietnia 2011 r. o zmianie ustawy o przeciwdziałaniu narkomanii ( Dz.U. 2011 nr 105 poz. 614 )". Internetowy System Aktów Prawnych. Retrieved 17 June 2011.
- "4-(Piperazin-1-yl)-2-(trifluoromethyl)phenol".
- "Erowid TFMPP Vault: Basics". Erowid. Retrieved 2014-02-15.
- ^ Schep LJ, Slaughter RJ, Vale JA, Beasley DM, Gee P (March 2011). "The clinical toxicology of the designer "party pills" benzylpiperazine and trifluoromethylphenylpiperazine". Clin Toxicol. 49 (3): 131–41. doi:10.3109/15563650.2011.572076. PMID 21495881. S2CID 42491343.
- ^ Baumann MH, Clark RD, Budzynski AG, Partilla JS, Blough BE, Rothman RB (March 2005). "N-substituted piperazines abused by humans mimic the molecular mechanism of 3,4-methylenedioxymethamphetamine (MDMA, or 'Ecstasy')". Neuropsychopharmacology. 30 (3): 550–60. doi:10.1038/sj.npp.1300585. PMID 15496938. S2CID 24217984.
- Robertson DW, Bloomquist W, Wong DT, Cohen ML (1992). "mCPP but not TFMPP is an antagonist at cardiac 5HT3 receptors". Life Sciences. 50 (8): 599–605. doi:10.1016/0024-3205(92)90372-V. PMID 1736030.
- Partilla JS, Dempsey AG, Nagpal AS, Blough BE, Baumann MH, Rothman RB (October 2006). "Interaction of amphetamines and related compounds at the vesicular monoamine transporter". Journal of Pharmacology and Experimental Therapeutics. 319 (1): 237–46. CiteSeerX 10.1.1.690.6669. doi:10.1124/jpet.106.103622. PMID 16835371. S2CID 22730478.
- Yarosh HL, Katz EB, Coop A, Fantegrossi WE (November 2007). "MDMA-like behavioral effects of N-substituted piperazines in the mouse". Pharmacology Biochemistry and Behavior. 88 (1): 18–27. doi:10.1016/j.pbb.2007.06.007. PMC 2082056. PMID 17651790.
- Wilkins C, Girling M, Sweetsur P, Huckle T, Huakau J. "Legal party pill use in New Zealand: Prevalence of use, availability, health harms and 'gateway effects' of benzylpiperazine (BZP) and trifluorophenylmethylpiperazine (TFMPP)" (PDF). Centre for Social and Health Outcomes Research and Evaluation (SHORE). Archived from the original (PDF) on 2007-03-18. Retrieved 2007-04-14.
- "SOR/2012-65 March 30, 2012 Controlled Drugs and Substances Act". Canada Gazette. Government of Canada. Retrieved 15 September 2014.
- "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
- "FINLEX ® - Säädökset alkuperäisinä: Valtioneuvoston asetus kuluttajamarkkinoilta… 1129/2015".
- "FINLEX ® - Säädökset alkuperäisinä: Valtioneuvoston asetus kuluttajamarkkinoilta… 225/2017".
- "FINLEX ® - Säädökset alkuperäisinä: Valtioneuvoston asetus kuluttajamarkkinoilta… 733/2021".
- "Misuse of Drugs (Classification of BZP) Amendment Bill 2008".
- "Erowid TFMPP Vault : Legal Status". Erowid.
- "RO 2010 4099". Fedlex. Retrieved 2022-08-16.
- "RO 2011 2595". Fedlex. Retrieved 2022-08-16.
- "§1308.11 Schedule I." Archived from the original on 2009-08-27. Retrieved 2014-12-17.
- "Scheduling Actions 2002". U.S. Department of Justice, Drug Enforcement Administration (DEA). Archived from the original on 2003-01-02.
- ^ Florida Statutes - Chapter 893 - DRUG ABUSE PREVENTION AND CONTROL
External links
| Empathogens/entactogens | |
|---|---|
| Phenylalkyl- amines (other than cathinones) |
|
| Cyclized phenyl- alkylamines | |
| Cathinones | |
| Tryptamines | |
| Chemical classes | |
| Monoamine releasing agents | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DRAsTooltip Dopamine releasing agents |
| ||||||||||||||
| NRAsTooltip Norepinephrine releasing agents |
| ||||||||||||||
| SRAsTooltip Serotonin releasing agents |
| ||||||||||||||
| Others |
| ||||||||||||||
| See also: Receptor/signaling modulators • Monoamine reuptake inhibitors • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
| Piperazines | |
|---|---|
| Simple piperazines (no additional rings) | |
| Phenylpiperazines |
|
| Benzylpiperazines | |
| Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines | |
| Pyridinylpiperazines | |
| Benzo(iso)thiazolylpiperazines | |
| Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized | |