| Revision as of 22:59, 5 November 2014 editZefr (talk | contribs)Extended confirmed users, Pending changes reviewers69,448 editsm →Effects of consumption by humans and other animals: repeat ref fix← Previous edit | Revision as of 03:20, 4 December 2014 edit undoRozo93 (talk | contribs)52 editsNo edit summaryNext edit → | ||
| Line 124: | Line 124: | ||
| === Quercetin release in the rutin degradation pathway === | === Quercetin release in the rutin degradation pathway === | ||
| The enzyme ] can be found in '']''.<ref name="urlInformation on EC 3.2.1.66 - quercitrinase">{{cite web | url = http://www.brenda-enzymes.org/php/result_flat.php4?ecno=3.2.1.66 | title = Information on EC 3.2.1.66 - quercitrinase | publisher = Helmholtz Centre for Infection Research | work = ] (BRaunschweig ENzyme DAtabase) }}</ref> Its substrates are ] and H<sub>2</sub>O and releases quercetin and L-]. It is an enzyme in the ] catabolic pathway<ref name="pmid20419500">{{cite journal | author = Tranchimand S, Brouant P, Iacazio G | title = The rutin catabolic pathway with special emphasis on quercetinase | journal = Biodegradation | volume = 21 | issue = 6 | pages = 833–59 | date = November 2010 | pmid = 20419500 | doi = 10.1007/s10532-010-9359-7 }}</ref> | The enzyme ] can be found in '']''.<ref name="urlInformation on EC 3.2.1.66 - quercitrinase">{{cite web | url = http://www.brenda-enzymes.org/php/result_flat.php4?ecno=3.2.1.66 | title = Information on EC 3.2.1.66 - quercitrinase | publisher = Helmholtz Centre for Infection Research | work = ] (BRaunschweig ENzyme DAtabase) }}</ref> Its substrates are ] and H<sub>2</sub>O and releases quercetin and L-]. It is an enzyme in the ] catabolic pathway<ref name="pmid20419500">{{cite journal | author = Tranchimand S, Brouant P, Iacazio G | title = The rutin catabolic pathway with special emphasis on quercetinase | journal = Biodegradation | volume = 21 | issue = 6 | pages = 833–59 | date = November 2010 | pmid = 20419500 | doi = 10.1007/s10532-010-9359-7 }}</ref> | ||
| === Conjugates === | |||
| ] is a human plasma quercetin metabolite. | |||
| === Glycosides === | === Glycosides === | ||
| Quercetin is the ] form of a number of other flavonoid ]s, such as ] and ], found in ] fruit, ] and onions. Quercetin forms the ]s ] and ] together with ] and ], respectively. Likewise ] is the 3-O-], ] is the 3-O-], ] is the 3-O-] and ] is the 4'-O-glucoside. ] is a quercetin derivative found in cottonseeds and cottonseed oil. ] is the quercetin 3-o-β-d-glucuronopyranoside.<ref name="pmid14735439">{{cite journal | author = Juergenliemk G, Boje K, Huewel S, Lohmann C, Galla HJ, Nahrstedt A | title = In vitro studies indicate that miquelianin (quercetin 3-o-β-d-glucuronopyranoside) is able to reach the CNS from the small intestine | journal = Planta Med. | volume = 69 | issue = 11 | pages = 1013–7 | date = November 2003 | pmid = 14735439 | doi = 10.1055/s-2003-45148 }}</ref> | Quercetin is the ] form of a number of other flavonoid ]s, such as ] and ], found in ] fruit, ] and onions. Quercetin forms the ]s ] and ] together with ] and ], respectively. Likewise ] is the 3-O-], ] is the 3-O-], ] is the 3-O-] and ] is the 4'-O-glucoside. ] is a quercetin derivative found in cottonseeds and cottonseed oil. ] is the quercetin 3-o-β-d-glucuronopyranoside.<ref name="pmid14735439">{{cite journal | author = Juergenliemk G, Boje K, Huewel S, Lohmann C, Galla HJ, Nahrstedt A | title = In vitro studies indicate that miquelianin (quercetin 3-o-β-d-glucuronopyranoside) is able to reach the CNS from the small intestine | journal = Planta Med. | volume = 69 | issue = 11 | pages = 1013–7 | date = November 2003 | pmid = 14735439 | doi = 10.1055/s-2003-45148 }}</ref> | ||
| ===Fate ''in vivo''=== | |||
| Following dietary ingestion, quercetin undergoes rapid and extensive metabolism that makes the biological effects presumed from in vitro studies unlikely to apply in vivo.<ref name=Williams>{{cite journal |author=Williams RJ, Spencer JP, Rice-Evans C |title=Flavonoids: antioxidants or signalling molecules? |journal=Free Radical Biology & Medicine |volume=36 |issue=7 |pages=838–49 |date=April 2004 |pmid=15019969 |doi=10.1016/j.freeradbiomed.2004.01.001}}</ref><ref name="roles">{{citation |title= New Roles for Polyphenols. A 3-Part report on Current Regulations & the State of Science |author= Gross P|date= March 1, 2009 |publisher= Nutraceuticals World |url= http://www.nutraceuticalsworld.com/issues/2009-03/view_features/new-roles-for-polyphenols/ }}</ref><ref>{{cite doi|10.1039/c1fo10025d}}</ref> | |||
| == Effects of consumption by humans and other animals == | == Effects of consumption by humans and other animals == | ||
Revision as of 03:20, 4 December 2014
| |
| Names | |
|---|---|
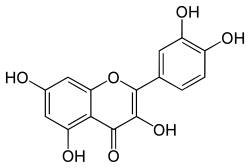
| IUPAC name 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one | |
| Other names
Sophoretin Meletin Quercetine Xanthaurine Quercetol Quercitin Quertine Flavin meletin | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.807 |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H10O7 |
| Molar mass | 302.236 g/mol |
| Appearance | yellow crystalline powder |
| Density | 1.799 g/cm |
| Melting point | 316 °C |
| Solubility in water | Practically insoluble in water; soluble in aqueous alkaline solutions |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |

Quercetin /ˈkwɜːrstn/ is a flavonol found in many fruits, vegetables, leaves and grains. It can be used as an ingredient in supplements, beverages, or foods.
Occurrence
Quercetin is a flavonoid widely distributed in nature. The name has been used since 1857, and is derived from quercetum (oak forest), after Quercus. It is a naturally occurring polar auxin transport inhibitor.
| Foods containing quercetin | Quercetin (mg/100g of edible portion) |
|---|---|
| capers, raw | 234 |
| capers, canned | 173 |
| lovage | 170 |
| dock like sorrel | 86 |
| radish leaves | 70 |
| carob fiber | 58 |
| dill | 55 (48-110) |
| cilantro | 53 |
| Hungarian wax pepper | 51 |
| fennel leaves | 48.8 |
| onion, red | 32 |
| radicchio | 31.5 |
| watercress | 30 |
| buckwheat | 23 |
| kale | 23 |
| chokeberry | 19 |
| cranberry | 15 |
| lingonberry | 13 |
| plums, black | 12 |
| cow peas | 11 |
| sweet potato | 10 |
| blueberry, cultivated | 8 |
| sea buckthorn berry | 8 |
| rowanberry | 7 |
| crowberry | 5 |
| prickly pear cactus fruits | 5 |
| apples, Red Delicious | 4 |
| broccoli | 3 |
| bilberry | 3 |
| tea, black or green Camellia sinensis | 2 |
In red onions, higher concentrations of quercetin occur in the outermost rings and in the part closest to the root, the latter being the part of the plant with the highest concentration. One study found that organically grown tomatoes had 79% more quercetin than chemically grown fruit. Quercetin is present in various kinds of honey from different plant sources.
Metabolism
Biosynthesis
Phenylalanine is converted to 4-coumaroyl-CoA in a series of steps known as the general phenylpropanoid pathway using phenylalanine ammonia-lyase, cinnamate-4-hydroxylase, and 4-coumaroyl-CoA-ligase. 4-Coumaroyl-CoA is added to three molecules of malonyl-CoA to form tetrahydroxychalcone using 7,2’-dihydroxy-4’-methoxyisoflavanol synthase. Tetrahydroxychalcone is then converted into naringenin using chalcone isomerase. Naringenin is then converted into eriodictyol using flavanoid 3’-hydroxylase. Eriodictyol is then converted into dihydroquercetin with flavanone 3-hydroxylase, which is then converted into quercetin using flavonol synthase.
Quercetin release in the rutin degradation pathway
The enzyme quercitrinase can be found in Aspergillus flavus. Its substrates are quercitrin and H2O and releases quercetin and L-rhamnose. It is an enzyme in the rutin catabolic pathway
Glycosides
Quercetin is the aglycone form of a number of other flavonoid glycosides, such as rutin and quercitrin, found in citrus fruit, buckwheat and onions. Quercetin forms the glycosides quercitrin and rutin together with rhamnose and rutinose, respectively. Likewise guaijaverin is the 3-O-arabinoside, hyperoside is the 3-O-galactoside, isoquercitin is the 3-O-glucoside and spiraeoside is the 4'-O-glucoside. CTN-986 is a quercetin derivative found in cottonseeds and cottonseed oil. Miquelianin is the quercetin 3-o-β-d-glucuronopyranoside.
Effects of consumption by humans and other animals
Quercetin itself (aglycone quercetin), as opposed to quercetin glycosides, is not a normal dietary component. In a bioavailability study in rats, radiolabelled quercetin-4'-glucoside was converted to phenolic acids as it passed through the gastrointestinal tract, producing compounds not monitored in previous animal studies of aglycone quercetin. All but 4% was recovered within 72 hours (69% in urine), indicating low retention and high excretion, a characteristic of ingested polyphenols. Quercetin may also induce insulin secretion by activation of L-type calcium channels in the pancreatic β-cells.
Quercetin has not been confirmed scientifically as a specific therapeutic for any condition nor approved by any regulatory agency. The European Food Safety Authority evaluated possible health claims associated with consumption of quercetin, finding that no cause-and-effect relationship has been established for any physiological effect.
Preliminary research
Although quercetin is under basic and early-stage clinical research for a variety of disease conditions, there exists no sufficient evidence that it has any beneficial effect in the human body. The US FDA has issued warning letters, e.g., to emphasize that quercetin is not a defined nutrient, cannot be assigned a dietary content level and is not regulated as a drug to treat any human disease.
Drug interactions
Quercetin is contraindicated with some antibiotics; it may interact with fluoroquinolones (an antibiotic), as quercetin competitively binds to bacterial DNA gyrase. Whether this inhibits or enhances the effect of fluoroquinolones is not certain.
AHFS Drug Information (2010) identifies quercetin as an inhibitor of CYP2C8, and specifically names it as a drug with potential to have harmful interactions with taxol/paclitaxel. As paclitaxel is metabolized primarily by CYP2C8, its bioavailability may be increased unpredictably, potentially leading to harmful side-effects.
See also
|
References
- ^ Quercetin dihydrate safety sheet on http://www.pvp.com.br (English)
- "Quercetin". Merriam-Webster.
- "Quercitin (biochemistry)". Encyclopædia Britannica.
- Fischer C, Speth V, Fleig-Eberenz S, Neuhaus G (October 1997). "Induction of Zygotic Polyembryos in Wheat: Influence of Auxin Polar Transport". Plant Cell. 9 (10): 1767–80. doi:10.1105/tpc.9.10.1767. PMC 157020. PMID 12237347.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "USDA Database for the Flavonoid Content of Selected Foods, Release 3.1" (PDF). U.S. Department of Agriculture. 2013.
- "Dill weed, fresh". Merschat family website.
- Justesen U, Knuthsen P (May 2001). "Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes". Food Chemistry. 73 (2): 245–50. doi:10.1016/S0308-8146(01)00114-5.
- ^ "USDA Database for the Flavonoid Content of Selected Foods, Release 3" (PDF). U.S. Department of Agriculture. 2011.
- "Food Nutrition Facts: Buckwheat". Merschat family website.
- Smith C, Lombard KA, Peffley EB, Liu W (2003). "Genetic Analysis of Quercetin in Onion (Allium cepa L.) Lady Raider" (PDF). The Texas Journal of Agriculture and Natural Resource. 16. Agriculture Consortium of Texas: 24–8. Archived from the original (PDF) on February 25, 2007.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: multiple names: authors list (link) - Mitchell AE, Hong YJ, Koh E, Barrett DM, Bryant DE, Denison RF, Kaffka S (July 2007). "Ten-year comparison of the influence of organic and conventional crop management practices on the content of flavonoids in tomatoes". J. Agric. Food Chem. 55 (15): 6154–9. doi:10.1021/jf070344. PMID 17590007.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Petrus K, Schwartz H, Sontag G (June 2011). "Analysis of flavonoids in honey by HPLC coupled with coulometric electrode array detection and electrospray ionization mass spectrometry". Anal Bioanal Chem. 400 (8): 2555–63. doi:10.1007/s00216-010-4614-7. PMID 21229237.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Winkel-Shirley B (June 2001). "Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology". Plant Physiol. 126 (2): 485–93. doi:10.1104/pp.126.2.485. PMC 1540115. PMID 11402179.
- "Information on EC 3.2.1.66 - quercitrinase". BRENDA (BRaunschweig ENzyme DAtabase). Helmholtz Centre for Infection Research.
- Tranchimand S, Brouant P, Iacazio G (November 2010). "The rutin catabolic pathway with special emphasis on quercetinase". Biodegradation. 21 (6): 833–59. doi:10.1007/s10532-010-9359-7. PMID 20419500.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Juergenliemk G, Boje K, Huewel S, Lohmann C, Galla HJ, Nahrstedt A (November 2003). "In vitro studies indicate that miquelianin (quercetin 3-o-β-d-glucuronopyranoside) is able to reach the CNS from the small intestine". Planta Med. 69 (11): 1013–7. doi:10.1055/s-2003-45148. PMID 14735439.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Mullen W, Rouanet JM, Auger C, Teissèdre PL, Caldwell ST, Hartley RC, Lean ME, Edwards CA, Crozier A (December 2008). "Bioavailability of quercetin-4'-glucoside in rats". J. Agric. Food Chem. 56 (24): 12127–37. doi:10.1021/jf802754s. PMID 19053221.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bardy G, Virsolvy A, Quignard JF, Ravier MA, Bertrand G, Dalle S, Cros G, Magous R, Richard S, Oiry C (July 2013). "Quercetin induces insulin secretion by direct activation of L-type calcium channels in pancreatic beta cells". Br. J. Pharmacol. 169 (5): 1102–13. doi:10.1111/bph.12194. PMC 3696332. PMID 23530660.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Scientific Opinion on the substantiation of health claims related to quercetin and protection of DNA, proteins and lipids from oxidative damage (ID 1647), "cardiovascular system" (ID 1844), "mental state and performance" (ID 1845), and "liver, kidneys" (ID 1846) pursuant to Article 13(1) of Regulation (EC) No 1924/2006". EFSA Journal. 8 April 2011. Retrieved 24 September 2014.
- Cite error: The named reference
roleswas invoked but never defined (see the help page). - "River Hills Harvest dba Elderberrylife". Adams, AM, Inspections, Compliance, Enforcement, and Criminal Investigations, US FDA. 22 April 2014. Retrieved 5 November 2014.
- Hilliard JJ, Krause HM, Bernstein JI, Fernandez JA, Nguyen V, Ohemeng KA, Barrett JF (1995). "A comparison of active site binding of 4-quinolones and novel flavone gyrase inhibitors to DNA gyrase". Adv. Exp. Med. Biol. 390: 59–69. doi:10.1007/978-1-4757-9203-4_5. PMID 8718602.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bun SS, Ciccolini J, Bun H, Aubert C, Catalin J (June 2003). "Drug interactions of paclitaxel metabolism in human liver microsomes". J Chemother. 15 (3): 266–74. doi:10.1179/joc.2003.15.3.266. PMID 12868554.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bun SS, Giacometti S, Fanciullino R, Ciccolini J, Bun H, Aubert C (July 2005). "Effect of several compounds on biliary excretion of paclitaxel and its metabolites in guinea-pigs". Anticancer Drugs. 16 (6): 675–82. doi:10.1097/00001813-200507000-00013. PMID 15930897.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- UMM Complementary and Alternative Medicine Guide: Quercetin (University of Maryland Medical Center Website)
| Antioxidants | |
|---|---|
| Food antioxidants |
|
| Fuel antioxidants | |
| Measurements | |
| Xenoestrogens | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phytoestrogens |
| ||||||||||||||||||||||||
| Mycoestrogens |
| ||||||||||||||||||||||||
| Synthetic | |||||||||||||||||||||||||
| Metalloestrogens | |||||||||||||||||||||||||
| Flavonols and their conjugates | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Backbone |
| ||||||||||||||||
| Flavonols |
| ||||||||||||||||
| O-Methylated flavonols |
| ||||||||||||||||
| Derivative flavonols |
| ||||||||||||||||
| Pyranoflavonols |
| ||||||||||||||||
| Furanoflavonols |
| ||||||||||||||||
| Semisynthetic |
| ||||||||||||||||