| Revision as of 20:17, 29 June 2016 editTom.Reding (talk | contribs)Autopatrolled, Extended confirmed users, Page movers, Template editors3,881,095 editsm →References: Rem stub tag(s) (class = non-stub & non-list) using AWB← Previous edit | Latest revision as of 18:46, 23 August 2024 edit undoKimen8 (talk | contribs)Extended confirmed users5,112 editsm →top: ce formatting | ||
| (29 intermediate revisions by 21 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Antidepressant}} | |||
| {{Drugbox | {{Drugbox | ||
| | Verifiedfields = changed | | Verifiedfields = changed | ||
| | Watchedfields = changed | |||
| | verifiedrevid = 416403788 | | verifiedrevid = 416403788 | ||
| | IUPAC_name = ''N''′-benzyl-5-methylisoxazole-3-carbohydrazide | | IUPAC_name = ''N''′-benzyl-5-methylisoxazole-3-carbohydrazide | ||
| Line 11: | Line 13: | ||
| | pregnancy_category = C (]) | | pregnancy_category = C (]) | ||
| | legal_AU = S4 | | legal_AU = S4 | ||
| | legal_BR = C1 | |||
| | legal_BR_comment = <ref>{{Cite web |author=Anvisa |author-link=Brazilian Health Regulatory Agency |date=2023-03-31 |title=RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial |trans-title=Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control|url=https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-784-de-31-de-marco-de-2023-474904992 |url-status=live |archive-url=https://web.archive.org/web/20230803143925/https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-784-de-31-de-marco-de-2023-474904992 |archive-date=2023-08-03 |access-date=2023-08-16 |publisher=] |language=pt-BR |publication-date=2023-04-04}}</ref> | |||
| | legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| | legal_DE = <!-- Anlage I, II, III or Unscheduled --> | |||
| | legal_NZ = <!-- Class A, B, C --> | |||
| | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | |||
| | legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| | legal_EU = | |||
| | legal_UN = <!-- N I, II, III, IV / P I, II, III, IV --> | |||
| | legal_status = Rx-only | | legal_status = Rx-only | ||
| | routes_of_administration = Oral | | routes_of_administration = Oral | ||
| <!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| | bioavailability = Low, peak at 1–2 h<ref name=isocoverview>{{cite book | vauthors = Owens DC, Johnstone EC, Lawrie SM | chapter = Clinical psychopharmacology. | title = Companion to psychiatric studies. | date = January 2010 | pages = 227–294 | doi = 10.1016/B978-0-7020-3137-3.00011-5 | isbn = 9780702031373 }}</ref> | |||
| | bioavailability = ? | |||
| | metabolism = ] (]<ref>{{cite journal | vauthors = Moroi K, Kuga T | title = Inhibitory effect of leptophos on carboxylesterase (isocarboxazid amidase) in rat liver | journal = Toxicology Letters | volume = 11 | issue = 1–2 | pages = 81–85 | date = April 1982 | pmid = 6178187 | doi = 10.1016/0378-4274(82)90110-2 }}</ref>) | |||
| | metabolism = ] | |||
| | elimination_half-life = |
| elimination_half-life = 1.5–4 h<ref name=isocoverview/> | ||
| | excretion = ] | | excretion = ] | ||
| | metabolites = ]<ref>{{cite web|url=https://go.drugbank.com/reactions/2399|website=go.drugbank.com|access-date=27 October 2021|title=Reaction: Isocarboxazid to 1 product}}</ref> | |||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | IUPHAR_ligand = 7204 | | IUPHAR_ligand = 7204 | ||
| Line 37: | Line 49: | ||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | C=12 | H=13 | N=3 | O=2 | | C=12 | H=13 | N=3 | O=2 | ||
| | molecular_weight = 231.25 g/mol | |||
| | smiles = O=C(NNCc1ccccc1)c2noc(c2)C | | smiles = O=C(NNCc1ccccc1)c2noc(c2)C | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| Line 45: | Line 56: | ||
| }} | }} | ||
| '''Isocarboxazid''' ('''Marplan''', '''Marplon''', '''Enerzer''') is a non-selective, ] ] (MAOI) of the ] class used as an ].<ref name="pmid2870717">{{cite journal |vauthors=Fagervall I, Ross SB |title=Inhibition of monoamine oxidase in monoaminergic neurones in the rat brain by irreversible inhibitors |journal= |
'''Isocarboxazid''' ('''Marplan''', '''Marplon''', '''Enerzer''') is a non-selective, ] ] (MAOI) of the ] class used as an ].<ref name="pmid2870717">{{cite journal | vauthors = Fagervall I, Ross SB | title = Inhibition of monoamine oxidase in monoaminergic neurones in the rat brain by irreversible inhibitors | journal = Biochemical Pharmacology | volume = 35 | issue = 8 | pages = 1381–1387 | date = April 1986 | pmid = 2870717 | doi = 10.1016/0006-2952(86)90285-6 }}</ref> Along with ] and ], it is one of only three classical MAOIs still available for clinical use in the treatment of psychiatric disorders in the ],<ref name="Rosenberg2013">{{cite book| vauthors = Rosenberg D |title=Pocket Guide For The Textbook Of Pharmacotherapy For Child And Adolescent Psychiatric Disorders|url=https://books.google.com/books?id=fep_AAAAQBAJ&pg=PA176|date=21 August 2013|publisher=Routledge|isbn=978-1-134-86002-9|pages=176–}}</ref><ref name="LabbateFava2012">{{cite book| vauthors = Labbate LA, Fava M, Rosenbaum JF, Arana GW |title=Handbook of Psychiatric Drug Therapy|url=https://books.google.com/books?id=xrZfcE8MydIC&pg=PA99|date=28 March 2012|publisher=Lippincott Williams & Wilkins|isbn=978-1-4511-5307-1|pages=99–}}</ref> though it is not as commonly employed in comparison to the others.<ref name="Rosenberg2013" /><ref name="LabbateFava2012" /> | ||
| Isocarboxazid is primarily used to treat ] and ]s. It has also been investigated in the treatment of ] |
Isocarboxazid is primarily used to treat ] and ]s. It has also been investigated in the treatment of ],<ref>{{cite journal | vauthors = Darling HF | title = Isocarboxazid (marplan) in ambulatory psychiatric patients | journal = The American Journal of Psychiatry | volume = 116 | issue = 4 | pages = 355–356 | date = October 1959 | pmid = 13814129 | doi = 10.1176/ajp.116.4.355 }}</ref> ] and other ]-related disorders.<ref>{{cite journal | vauthors = Riederer P, Laux G | title = MAO-inhibitors in Parkinson's Disease | journal = Experimental Neurobiology | volume = 20 | issue = 1 | pages = 1–17 | date = March 2011 | pmid = 22110357 | pmc = 3213739 | doi = 10.5607/en.2011.20.1.1 | publisher = Experimental Neurology }}</ref> | ||
| Isocarboxazid, as well as other MAOIs, increase the levels of the ] ], ], ], ], ], ] in the brain.<ref name="pmid9829163">{{cite journal | vauthors = Volz HP, Gleiter CH | title = Monoamine oxidase inhibitors. A perspective on their use in the elderly | journal = Drugs & Aging | volume = 13 | issue = 5 | pages = 341–55 | date = November 1998 | pmid = 9829163 | doi = 10.2165/00002512-199813050-00002 | s2cid = 71158339 }}</ref> | |||
| Classical MAOIs, including isocarboxazid, are used only |
Classical MAOIs, including isocarboxazid, are used only rarely due to prominent ] and ]s and have been largely superseded by newer antidepressants such as the ]s (SSRIs). The cause of the interactions is because MAOIs inhibit the ] of dietary amines (e.g., ]) and the monoamine neurotransmitters. In combination with other drugs that increase the levels of the monoamine neurotransmitters such as the SSRIs, or with certain foods high in dietary amines such as ]s, MAOIs can produce dangerous elevations of monoamine neurotransmitters resulting in potentially life-threatening syndromes such as ] and ]. | ||
| == See also == | == See also == | ||
| Line 58: | Line 71: | ||
| {{Antidepressants}} | {{Antidepressants}} | ||
| {{Monoamine metabolism modulators}} | |||
| {{Adrenergics}} | |||
| {{Hydrazines}} | {{Hydrazines}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | ] | ||
Latest revision as of 18:46, 23 August 2024
Antidepressant Pharmaceutical compound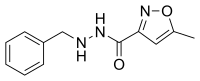 | |
| Clinical data | |
|---|---|
| Trade names | Marplan |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a605036 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Low, peak at 1–2 h |
| Metabolism | Liver (Carboxylesterase) |
| Metabolites | Hippuric acid |
| Elimination half-life | 1.5–4 h |
| Excretion | Urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.399 |
| Chemical and physical data | |
| Formula | C12H13N3O2 |
| Molar mass | 231.255 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Isocarboxazid (Marplan, Marplon, Enerzer) is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class used as an antidepressant. Along with phenelzine and tranylcypromine, it is one of only three classical MAOIs still available for clinical use in the treatment of psychiatric disorders in the United States, though it is not as commonly employed in comparison to the others.
Isocarboxazid is primarily used to treat mood and anxiety disorders. It has also been investigated in the treatment of schizophrenia, Parkinson's disease and other dementia-related disorders.
Isocarboxazid, as well as other MAOIs, increase the levels of the monoamine neurotransmitters serotonin, dopamine, norepinephrine, epinephrine, melatonin, phenethylamine in the brain.
Classical MAOIs, including isocarboxazid, are used only rarely due to prominent food and drug interactions and have been largely superseded by newer antidepressants such as the selective serotonin reuptake inhibitors (SSRIs). The cause of the interactions is because MAOIs inhibit the metabolism of dietary amines (e.g., tyramine) and the monoamine neurotransmitters. In combination with other drugs that increase the levels of the monoamine neurotransmitters such as the SSRIs, or with certain foods high in dietary amines such as aged cheeses, MAOIs can produce dangerous elevations of monoamine neurotransmitters resulting in potentially life-threatening syndromes such as hypertensive crisis and serotonin syndrome.
See also
References
- "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Owens DC, Johnstone EC, Lawrie SM (January 2010). "Clinical psychopharmacology.". Companion to psychiatric studies. pp. 227–294. doi:10.1016/B978-0-7020-3137-3.00011-5. ISBN 9780702031373.
- "Reaction: Isocarboxazid to 1 product". go.drugbank.com. Retrieved 27 October 2021.
- Moroi K, Kuga T (April 1982). "Inhibitory effect of leptophos on carboxylesterase (isocarboxazid amidase) in rat liver". Toxicology Letters. 11 (1–2): 81–85. doi:10.1016/0378-4274(82)90110-2. PMID 6178187.
- Fagervall I, Ross SB (April 1986). "Inhibition of monoamine oxidase in monoaminergic neurones in the rat brain by irreversible inhibitors". Biochemical Pharmacology. 35 (8): 1381–1387. doi:10.1016/0006-2952(86)90285-6. PMID 2870717.
- ^ Rosenberg D (21 August 2013). Pocket Guide For The Textbook Of Pharmacotherapy For Child And Adolescent Psychiatric Disorders. Routledge. pp. 176–. ISBN 978-1-134-86002-9.
- ^ Labbate LA, Fava M, Rosenbaum JF, Arana GW (28 March 2012). Handbook of Psychiatric Drug Therapy. Lippincott Williams & Wilkins. pp. 99–. ISBN 978-1-4511-5307-1.
- Darling HF (October 1959). "Isocarboxazid (marplan) in ambulatory psychiatric patients". The American Journal of Psychiatry. 116 (4): 355–356. doi:10.1176/ajp.116.4.355. PMID 13814129.
- Riederer P, Laux G (March 2011). "MAO-inhibitors in Parkinson's Disease". Experimental Neurobiology. 20 (1). Experimental Neurology: 1–17. doi:10.5607/en.2011.20.1.1. PMC 3213739. PMID 22110357.
- Volz HP, Gleiter CH (November 1998). "Monoamine oxidase inhibitors. A perspective on their use in the elderly". Drugs & Aging. 13 (5): 341–55. doi:10.2165/00002512-199813050-00002. PMID 9829163. S2CID 71158339.
| Monoamine metabolism modulators | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-specific |
| ||||||||||
| Phenethylamines (dopamine, epinephrine, norepinephrine) |
| ||||||||||
| Tryptamines (serotonin, melatonin) |
| ||||||||||
| Histamine |
| ||||||||||
| See also: Receptor/signaling modulators • Adrenergics • Dopaminergics • Melatonergics • Serotonergics • Monoamine reuptake inhibitors • Monoamine releasing agents • Monoamine neurotoxins | |||||||||||
| Hydrazines | |
|---|---|
|