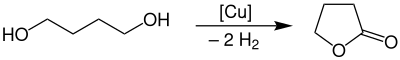| Revision as of 08:00, 9 August 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'DrugBank', 'ChEBI').← Previous edit | Latest revision as of 08:32, 30 December 2024 edit undo146.198.215.95 (talk) →Uses | ||
| (394 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{cs1 config|name-list-style=vanc|display-authors=6}} | |||
| {{DISPLAYTITLE:''gamma''-Butyrolactone}} | |||
| {{lowercase title}} | |||
| {{chembox | {{chembox | ||
| | verifiedrevid = 443831866 | |||
| | Watchedfields = changed | |||
| | Reference = <ref>'']'', 12th edition, '''1632'''.</ref><ref name=CRC90>{{cite book | veditors = Lide DR |title=CRC Handbook of Chemistry and Physics |url=http://www.crcpress.com/product/isbn/9781420090840 |publisher=CRC Press |location=Boca Raton, FL |edition=90th |date=2009-06-03 |access-date=2011-07-18 |isbn=978-1-4200-9084-0 |archive-date=2011-07-16 |archive-url=https://web.archive.org/web/20110716073708/http://www.crcpress.com/product/isbn/9781420090840 |url-status=dead }}</ref> | |||
| | verifiedrevid = 409743882 | |||
| | Name = γ-Butyrolactone | |||
| | Reference=<ref>'']'', 12th Edition, '''1632'''.</ref><ref name=CRC90>{{cite book|editor-last=Lide|editor-first=David R.|title=]|edition=90th|date=2009-06-03|publisher=CRC Press|location=Boca Raton, Florida|isbn=1420090844|url=http://www.crcpress.com/product/isbn/9781420090840|accessdate=2011-07-18}}</ref> | |||
| | ImageFile1_Ref = {{chemboximage|correct|??}} | |||
| | Name = ''gamma''-Butyrolactone | |||
| | ImageFile = | |||
| | ImageFile1_Ref = {{chemboximage|correct|??}} | |||
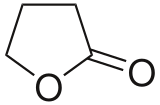
| | ImageFile1 = Gamma-Butyrolactone.svg | | ImageFile1 = Gamma-Butyrolactone.svg | ||
| | |
| ImageSize1 = 160 | ||
| | |
| ImageFile2 = GBL-from-xtal-3D-balls.png | ||
| | |
| ImageSize2 = | ||
| | |
| PIN = Oxolan-2-one | ||
| | SystematicName = | |||
| | OtherNames = GBL, butyrolactone, 1,4-lactone, 4-butyrolactone, 4-hydroxybutyric acid lactone, gamma-hydroxybutyric acid lactone, and oxolan-2-one | |||
| | OtherNames = Dihydrofuran-2(3''H'')-one<br />GBL<br />Butyrolactone<br />1,4-Lactone<br />4-Butyrolactone<br />4-Hydroxybutyric acid lactone<br />gamma-Hydroxybutyric acid lactone | |||
| | IUPACName = | |||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | IUPHAR_ligand = 5462 | |||
| | DrugBank = DB04699 | |||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | DrugBank = DB04699 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 42639 | | ChEBI = 42639 | ||
| | SMILES = O=C1OCCC1 | | SMILES = O=C1OCCC1 | ||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 7029 | | ChemSpiderID = 7029 | ||
| | PubChem = 7302 | | PubChem = 7302 | ||
| Line 33: | Line 39: | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo = 96-48-0 | | CASNo = 96-48-0 | ||
| | |
| RTECS = LU3500000 | ||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | |
| C=4 | H=6 | O=2 | ||
| | Appearance = Colorless liquid | |||
| | H = 6 | |||
| | Odor = Weak characteristic odor, comparable to stale water, synthetic melon aroma or burnt plastic | |||
| | O = 2 | |||
| | Density = 1.1286{{nbsp}}g/mL (15{{nbsp}}°C), <!-- From CRC90: -->1.1296{{nbsp}}g/mL (20{{nbsp}}°C) | |||
| | MassRound = 3 | |||
| | Solubility = Miscible | |||
| | Appearance = Colorless oily liquid | |||
| | SolubleOther = Soluble in ], ], <!-- Listed as "very soluble" in CRC90: --> ], ], ], ] | |||
| | Density = 1.1286 g/mL (15 °C), <!-- From CRC90: --> 1.1296 g/mL (20 °C) | |||
| | MeltingPtC = -43.53 | |||
| | Solubility = Miscible | |||
| | BoilingPtC = 204 | |||
| | SolubleOther = soluble in ], ], <!-- Listed as "very soluble" in CRC90: --> ], ], ], ] | |||
| | |
| pKa = 4.5 | ||
| | VaporPressure = 1.5 kPa (20{{nbsp}}°C) | |||
| | BoilingPtC = 204 | |||
| | Viscosity = 1.7{{nbsp}}cP (25{{nbsp}}°C) | |||
| | pKa = 4.5 | |||
| | |
| RefractIndex = 1.435, <!-- From CRC90: -->1.4341 (20{{nbsp}}°C) | ||
| | LogP = −0.76<ref name="chemsrc">{{Cite web|url=https://www.chemsrc.com/en/cas/96-48-0_895963.html|title=gamma-Butyrolactone|website=www.chemsrc.com}}</ref> | |||
| | RefractIndex = 1.435, <!-- From CRC90: --> 1.4341 (20 °C) | |||
| }} | }} | ||
| | Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| | |
| Dipole = | ||
| }} | }} | ||
| | Section4 = | |||
| | Section5 = | |||
| | Section6 = | |||
| | Section7 = {{Chembox Hazards | | Section7 = {{Chembox Hazards | ||
| | GHSSignalWord = Danger | |||
| | ExternalMSDS = | |||
| | ExternalSDS = | |||
| | MainHazards = Harmful | |||
| | MainHazards = Toxic and flammable | |||
| | FlashPt = 98 °C (closed cup) | |||
| | FlashPtC = 98 | |||
| | RPhrases = {{R22}} {{R36}} | |||
| | AutoignitionPtC = 455 | |||
| | SPhrases = {{S26}} {{S36}} | |||
| | |
| ExploLimits = 3.6% v/v (lower)<br/>16% v/v (upper) | ||
| | FlashPt_notes = (closed cup) | |||
| }} | |||
| | HPhrases = {{H-phrases|318|302|336}} | |||
| | Section8 = {{Chembox Related | |||
| | PPhrases = {{P-phrases|264|270|280|301+312|305+351+338|403+233|501}} | |||
| | OtherCpds = | |||
| | GHSPictograms = {{GHS05}}{{GHS07}} | |||
| | LD50 = 1540 mg/kg (oral, rat)<br/>>5640 mg/kg (dermal, rabbit) | |||
| | LC50 = >2.68 mg/kg (rat, 4h) | |||
| | NFPA-H = 2 | |||
| | NFPA-F = 1 | |||
| | NFPA-I = 1 | |||
| }} | }} | ||
| |Section8={{Chembox Legal status | |||
| | legal_AU = | |||
| | legal_BR = B1 | |||
| | legal_BR_comment = <ref>{{Cite web |author=Anvisa |author-link=Brazilian Health Regulatory Agency |date=2023-03-31 |title=RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial |trans-title=Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control|url=https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-784-de-31-de-marco-de-2023-474904992 |url-status=live |archive-url=https://web.archive.org/web/20230803143925/https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-784-de-31-de-marco-de-2023-474904992 |archive-date=2023-08-03 |access-date=2023-08-16 |publisher=] |language=pt-BR |publication-date=2023-04-04}}</ref> | |||
| | legal_US = | |||
| | legal_UK = | |||
| | legal_UN = | |||
| }} | |||
| | Section9 = {{Chembox Related | |||
| | OtherCompounds = | |||
| }} | |||
| }} | }} | ||
| '''γ-Butyrolactone''' ('''GBL''') or '''''gamma''-butyrolactone''' is an ] with the formula {{chem2|O\dCO(CH2)3}}. It is a ], colorless, water-miscible liquid with a weak characteristic ]. It is the simplest ]. It is mainly used as an intermediate in the production of other chemicals, such as ].<ref name=Ullmann/> | |||
| In humans, GBL acts as a ] for ] (GHB) and is often used as a ]. GHB acts as a ] (CNS) ] with effects similar to those of ].<ref>{{cite journal | vauthors = Schep LJ, Knudsen K, Slaughter RJ, Vale JA, Mégarbane B | title = The clinical toxicology of γ-hydroxybutyrate, γ-butyrolactone and 1,4-butanediol | journal = Clinical Toxicology | volume = 50 | issue = 6 | pages = 458–470 | date = July 2012 | pmid = 22746383 | doi = 10.3109/15563650.2012.702218 | s2cid = 19697449 }}</ref> | |||
| '''''gamma''-Butyrolactone''' (γ-butyrolactone or GBL) is a ] colorless oily liquid with a weak characteristic ] and is soluble in water. GBL is a common ] and ] in ] and is used as an ], as a ], as a ] remover, as a ], and as a ] in some wet aluminium electrolytic capacitors. In humans it acts as a ] for ], and it is used as a recreational intoxicant. | |||
| ==Occurrence== | ==Occurrence== | ||
| GBL has been found in extracts from samples of unadulterated wines.<ref name="pmid11569560">{{cite journal | |
GBL has been found in extracts from samples of unadulterated wines.<ref name="pmid11569560">{{cite journal | vauthors = Vose J, Tighe T, Schwartz M, Buel E | title = Detection of gamma-butyrolactone (GBL) as a natural component in wine | journal = Journal of Forensic Sciences | volume = 46 | issue = 5 | pages = 1164–1167 | date = September 2001 | pmid = 11569560 | doi = 10.1520/JFS15116J }}</ref><ref>{{cite journal | vauthors = Elliott S, Burgess V | title = The presence of gamma-hydroxybutyric acid (GHB) and gamma-butyrolactone (GBL) in alcoholic and non-alcoholic beverages | journal = Forensic Science International | volume = 151 | issue = 2–3 | pages = 289–292 | date = July 2005 | pmid = 15939164 | doi = 10.1016/j.forsciint.2005.02.014 }}</ref> This finding indicates that GBL is a naturally occurring component in some ]s and may be present in similar products. The concentration detected was approximately 5 μg/mL and was easily observed using a simple extraction technique followed by ] analysis. GBL can be found in cheese flavorings but typically results in a content of 0.0002% GBL in the final foodstuff.<ref name="HO circular"/> | ||
| ==Production and synthesis== | |||
| ==Preparation== | |||
| γ-Butyrolactone is produced industrially by dehydrogenation of ] at a temperature of 180–300 °C and atmospheric pressure in the presence of a copper catalyst.<ref name=Ullmann>{{Ullmann | vauthors = Schwarz W, Schossig J, Rossbacher R, Pinkos R, Höke H |title=Butyrolactone |year=2019 |doi=10.1002/14356007.a04_495.pub2 |isbn=978-3527306732}}</ref> | |||
| GBL can be synthesized from ] (GHB) by removal of water or by distillation from such a mixture. It may also be obtained via oxidation of ] (THF). One such process, which affords GBL in yields of up to 80%, utilises ] generated '']'' from an aqueous solution of ] and ].<ref>Metsger, L.; Bittner, S. Autocatalytic Oxidation of Ethers with Sodium Bromate, ''Tetrahedron'' '''2000''', 56, 1905-1910</ref> Another process can proceed by using commercially-available ] in the presence of activating ] and an appropriate solvent such as acetonitrile. Y-aminobutryic acid (GABA) can also be converted into GBL via | |||
| a quite simple Sandmeyer Reaction: with the addition of sodium nitrite and then acidified with an appropriate acid like HCl(aq). The acidification allows the sodium nitrite to act as nitrous acid, in laymen's terms, and through an intermediary reaction (converting GABA, an amine, to its diazatonium salt, which is then hydrolyzed leaving behind a hydroxyl group where the amino group was) form Y-Butyrolactone. The resulting GBL is simply combined with a stoichometricly correct amount of NaOH resulting in NaGHB; aka sodium Y-hydroxybutyrate. | |||
| :] | |||
| ==Chemistry== | |||
| GBL is a ]. It is ] under ] conditions, for example in a ] ] into ], the ] ] of ]. Under acidic conditions it forms an ] mixture of both compounds. These compounds then may go on to form a ]. | |||
| The yield of this process is approximately 95%. The purification takes place with a liquid-gas-phase extraction.<ref name="Ullmann"/> | |||
| In the laboratory, it may also be obtained via the oxidation of ] (THF), for example with aqueous ].<ref>{{cite journal | vauthors = Metsger L, Bittner S |title=Autocatalytic Oxidation of Ethers with Sodium Bromate |journal=Tetrahedron |volume=56 |issue=13 |pages=1905–1910 |date=March 2000 |doi=10.1016/S0040-4020(00)00098-3}}</ref> An alternative route proceeds from ] via a ].<ref>{{cite web |url=https://erowid.org/archive/rhodium/chemistry/gaba2ghb.html |title=Sandmeyer Reaction of GABA to GBL/GHB |access-date=2018-06-14}}</ref> | |||
| ==Reactions== | |||
| As a ], GBL is ] under ] conditions, for example in a ] ] into ], the ] ] of ]. In acidic water, a mixture of the lactone and acid forms exists in an ]. These compounds then may go on to form the ] ] as well as the ] ]. When treated with a non-nucleophilic base, such as ], GBL undergoes deprotonation of the alpha carbon atom to the carbonyl. The related compound ] can be used to make a polyester in this manner. | |||
| === Polymerization === | |||
| The ] of butyrolactone gives polybutyrolactone. The resulting reverts to the monomer by thermal ].<ref name="Micu">{{cite web | vauthors = Micu A |title=New, fully recyclable and biodegradable plastic could change the world |url=http://www.zmescience.com/science/recyclable-degradable-plastic-363454 |publisher=ZME Science |date=December 12, 2015 |access-date=2015-12-13 |language=en-US}}</ref><ref>{{cite journal | vauthors = Hong M, Chen EY | title = Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of γ-butyrolactone | journal = Nature Chemistry | volume = 8 | issue = 1 | pages = 42–49 | date = January 2016 | pmid = 26673263 | doi = 10.1038/nchem.2391 }}</ref> It is claimed that poly(GBL) is competitive with commercial biomaterial poly(4-]), or P4HB. It is further claimed that poly(GBL) is cheaper to make than P4HB, although both are bio-derived.<ref name="Micu"/><ref name=hong>{{cite journal | vauthors = Hong M, Chen EY | title = Towards Truly Sustainable Polymers: A Metal-Free Recyclable Polyester from Biorenewable Non-Strained γ-Butyrolactone | journal = Angewandte Chemie | volume = 55 | issue = 13 | pages = 4188–4193 | date = March 2016 | pmid = 26934184 | doi = 10.1002/anie.201601092 }}</ref> | |||
| ==Uses== | |||
| Gamma-Butyrolactone is used as a chemical solvent and a cleaning agent,<ref>{{cite web |title=Gamma-Gamma-Butyrolactone:an industrial solvent |url=https://www.chemicalbook.com/article/gamma-butyrolactone-an-industrial-solvent.htm |website=Chemical Book |access-date=21 June 2024}}</ref> for example in paint stripping or for cleaning ].<ref>{{cite web |title =Insider tell you why the GBL Cleaner is the best solution for these nasty dirties and stains | |||
| |url=https://medium.com/@pressreleaserwe/insider-tell-you-why-the-gbl-cleaner-is-the-best-solution-for-these-nasty-dirties-and-stains-8044d078c8 |website=Medium |date=16 December 2014 |access-date=21 June 2024}}</ref> | |||
| Butyrolactone is a precursor to other chemicals. Reaction with methylamine gives ], and with ammonia gives ]. It is also used as a solvent in lotions and some polymers.<ref name=Ullmann/> | |||
| [[Image:MCPB.png|thumb|left|243px|[[MCPB|2-Methyl-4-chlorophenoxybutyric | |||
| acid]] is an herbicide produced from butyrolactone.]] | |||
| Butyrolactone, with its wide liquid range, chemical stability, and high ], is used in ] as the organic solvent. It is frequently mixed with a small ratio of ethylene glycol, "9:1" being common, to vary internal ].{{citation needed|date=June 2024}} | |||
| It has been used as a solvent in various laboratory experiments, e.g., the preparation of ].<ref>{{cite journal | doi=10.1002/adma.201706576 | title=Stable High-Performance Perovskite Solar Cells via Grain Boundary Passivation | date=2018 | journal=Advanced Materials | volume=30 | issue=16 | pmid=29527750 | vauthors = Niu T, Lu J, Munir R, Li J, Barrit D, Zhang X, Hu H, Yang Z, Amassian A, Zhao K, Liu S }}</ref> | |||
| GBL is used in the synthesis of ], ], ],<ref>Anton Ebnother, Ernst Jucker, Erwin Rissi, {{US patent|3531480}} (1970 to Sandoz Ag).</ref> ] ,<ref>EP0932597 idem Roberto Cacciaglia, et al. WO1998006690 (Laboratorio Farmaceutico CT SRL).</ref><ref>Roberto Cacciaglia & Massimo Ferrari, WO2009062949 (Laboratorio Farmaceutico CT SRL).</ref> ],<ref>Sydney Archer, US3562278 & US3639414 (1971 to Sterling Drug Inc).</ref> & ] (page 1451 cmp. 72).<ref>Maryanoff BE, McComsey DF, Gardocki JF, Shank RP, Costanzo MJ, Nortey SO, et al. (August 1987). "Pyrroloisoquinoline antidepressants. 2. In-depth exploration of structure-activity relationships". Journal of Medicinal Chemistry. 30 (8): 1433–54. doi:10.1021/jm00391a028. PMID 3039136.</ref> | |||
| GBL is known to be used in the synthesis of 4-chlorobutyryl chloride.<ref>CN101445447A</ref> This in turn is used in the synthesis of the butyrophenone sidechain of many antipsychotic drugs (e.g. haloperidol). | |||
| It is also known to be employed in the synthesis of 1-bromo-4,4-bis(p-fluorophenyl)butane <ref>Junying Yuan, et al. WO2011143444 ().</ref> (the side-chain to the ] agents). | |||
| from GBL]] | |||
| It was claimed that the reaction of GBL with benzene in the presence of aluminium trichloride could furnish ] in a single step.<ref>{{cite journal | doi=10.15227/orgsyn.035.0095 | title=A-TETRALONE | journal=Organic Syntheses | date=1955 | volume=35 | page=95 }}</ref> | |||
| Another discovered GBL utility is in the synthesis of nicotine (analogs):<ref>{{cite journal | doi=10.1002/jhet.233 | title=A new and efficient approach to the synthesis of nicotine and anabasine analogues | date=2009 | journal=Journal of Heterocyclic Chemistry | volume=46 | issue=6 | pages=1252–1258 | pmid=20161612 | pmc=2811585 | vauthors = Huang K, Ortiz-Marciales M, De Jesús M, Stepanenko V }}</ref> | |||
| ==Pharmacology== | ==Pharmacology== | ||
| {{ |
{{main|gamma-hydroxybutyrate#Pharmacology}} | ||
| GBL is not active in its own right; its mechanism of action stems from its identity as a ] of GHB. | GBL is not active in its own right; its mechanism of action stems from its identity as a ] of GHB. | ||
| The hypnotic effect of GHB is enhanced by combination with alcohol. A 2003 rat study showed that GBL in combination with ethanol showed a potentiated hypnotic effect, as the sleep-timing measure was longer than both of the individual components combined.<ref> | |||
| {{Cite journal | |||
| | last = Sassenbroek | |||
| | first = Van | |||
| | title = Characterization of the pharmacokinetic and pharmacodynamic interaction between gamma-hydroxybutyrate and ethanol in the rat. | |||
| | journal = ] | |||
| | date = June 2007 | |||
| | pmid = 12700396 | |||
| | last2 = De Paepe | |||
| | first2 = P | |||
| | last3 = Belpaire | |||
| | first3 = FM | |||
| | last4 = Buylaert | |||
| | first4 = WA | |||
| | volume = 73 | |||
| | issue = 2 | |||
| | pages = 270–8 | |||
| | doi = 10.1093/toxsci/kfg079 | |||
| | postscript = <!--None--> }}</ref> | |||
| ===Pharmacokinetics=== | ===Pharmacokinetics=== | ||
| GBL is rapidly converted into GHB by ] (lactonase) enzymes, found in the blood.<ref name="pmid2030821">{{cite journal | vauthors = Snead OC | title = The gamma-hydroxybutyrate model of absence seizures: correlation of regional brain levels of gamma-hydroxybutyric acid and gamma-butyrolactone with spike wave discharges | journal = Neuropharmacology | volume = 30 | issue = 2 | pages = 161–167 | date = February 1991 | pmid = 2030821 | doi = 10.1016/0028-3908(91)90199-l }}</ref><ref name="Forchem">{{cite book | vauthors = Kobilinsky L |title=Forensic Chemistry Handbook |url=https://books.google.com/books?id=m_vIAgAAQBAJ |date=2011-11-29 |page=386 |publisher=John Wiley & Sons |isbn=978-0-471-73954-8}}</ref><ref>{{cite journal | vauthors = Teiber JF, Draganov DI, La Du BN | title = Lactonase and lactonizing activities of human serum paraoxonase (PON1) and rabbit serum PON3 | journal = Biochemical Pharmacology | volume = 66 | issue = 6 | pages = 887–896 | date = September 2003 | pmid = 12963475 | doi = 10.1016/S0006-2952(03)00401-5 }}</ref> Animals which lack these enzymes exhibit no effect from GBL.<ref name="Forchem"/> GBL is more lipophilic (fat soluble) than GHB, and so is absorbed faster and has higher ]. Because of these pharmacokinetic differences, GBL tends to be more potent and faster-acting than GHB, but has a shorter duration; whereas the related compound ] (1,4-B) tends to be slightly less potent and slower to take effect but longer-acting than GHB.<ref name="WHO report">{{cite web |title=Gamma-butyrolactone (GBL) Pre-Review Report |url=https://www.who.int/medicines/areas/quality_safety/5.4GBLpre-review.pdf |date=4 June 2012}}</ref> | |||
| {{Citations missing|section|date=April 2011|DO NOT REFERENCE INTERNET FORUMS}} | |||
| GBL is rapidly converted into GHB by lactonase enzymes found in the blood. GBL is more lipophilic (fat soluble) than GHB, and so is absorbed faster and has higher ]; the paradox is that this can mean that GBL has a faster onset of effects than GHB itself, even though it is a prodrug. The levels of lactonase enzyme can vary between individuals, meaning that first-time users can show unpredictable results, even from small doses. In many this manifests as slow onset of effects, followed by headaches, semi-consciousness which is distinct from GBL sleep in normal users. If the user decides to try again at a later date, they appear to be able to enjoy the effects normally. Because of these pharmacokinetic differences, GBL tends to be more potent and faster-acting than GHB, but has a shorter duration; whereas the related compound ] (1,4-B) tends to be slightly less potent, slower to take effect but longer-acting than GHB. | |||
| ], GBL and ] |
], GBL and ]]] | ||
| == |
===Nutritional supplement=== | ||
| {{main|Gamma-Hydroxybutyric acid#Sports and athletics}} | |||
| Due to its property of being a ] of ], GBL was sold as a nutritional supplement after the scheduling of GHB, under the names Revivarant and Renewtrient in the U.S. at least until the end of 1999. | |||
| Due to its property of being a ] of ] which increases sleep related growth hormone (GH) secretion,<ref>{{cite journal | vauthors = Van Cauter E, Plat L, Scharf MB, Leproult R, Cespedes S, L'Hermite-Balériaux M, Copinschi G | title = Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men | journal = The Journal of Clinical Investigation | volume = 100 | issue = 3 | pages = 745–753 | date = August 1997 | pmid = 9239423 | pmc = 508244 | doi = 10.1172/JCI119587 }}</ref> GBL was sold as a nutritional supplement after the scheduling of GHB, under the names ''Revivarant'' and ''Renewtrient'',<ref name='erowid rev'>{{cite web |title=Erowid GHB vault: FDA Warning about Gamma Butyrlactone |url=https://www.erowid.org/chemicals/ghb/gbl_info2.shtml |publisher=] |date=1998-11-21 |access-date=2013-10-10}}</ref> until they were banned by the FDA. | |||
| ===Recreational drug=== | |||
| GBL (as well as GHB), when taken internally in therapeutic doses without the presence of other drugs (especially alcohol, as mixing the two can be fatal), has been shown to elevate growth hormone levels in humans to at least 5 times the baseline. {{Citation needed|date=October 2009}} | |||
| GBL is a ] of ] (naturally produced) and its recreational use comes entirely as a result of this.<ref>{{cite book | vauthors = Meyer J, Quenzer LF |title=Psychopharmacology: Drugs, the Brain and Behavior |title-link=Psychopharmacology |publisher=Sinauer |year=2005 |page=370 |isbn=978-0-87893-534-5}}</ref> GBL overdose can cause irrational behavior, severe sickness, ] and ].<ref>{{cite web |title=USDOJ: U.S. Department of Justice Archive National Drug Intelligence Center |url=http://www.usdoj.gov/ndic/pubs4/4532/4532p.pdf |publisher=Usdoj.gov |date=2012-06-15 |access-date=2014-01-22}}</ref> | |||
| To bypass GHB restriction laws, home synthesis kits were introduced to transform GBL and/or 1,4-B into GHB. | |||
| ==Recreational use== | |||
| ] | |||
| GBL is a ] of ]. To bypass GHB restriction laws, home synthesis kits were introduced to transform GBL and/or 1,4-B into GHB. GBL can also be used as a recreational drug by itself.<ref> | |||
| ] | |||
| {{cite book | |||
| GBL has a distinctive taste and odor, described as being comparable to stale water, synthetic melon aroma or burnt plastic. This differs significantly from GHB, which is described as having a decidedly "salty" taste.<ref>{{cite journal | vauthors = Galloway GP, Frederick-Osborne SL, Seymour R, Contini SE, Smith DE | title = Abuse and therapeutic potential of gamma-hydroxybutyric acid | journal = Alcohol | volume = 20 | issue = 3 | pages = 263–269 | date = April 2000 | pmid = 10869868 | doi = 10.1016/S0741-8329(99)00090-7 }}</ref> | |||
| | last = Meyer | |||
| | first = Jerrold | |||
| | coauthors = Linda F. Quenzer | |||
| | title = ]: Drugs, the Brain and Behavior | |||
| | publisher = Sinauer | |||
| | year = 2005 | |||
| | isbn = 0-87893-534-7 | |||
| | page = 370 }} | |||
| </ref> | |||
| ] | |||
| ] | |||
| GBL overdose can cause irrational behaviour, severe sickness, ] and ]. <ref>http://www.usdoj.gov/ndic/pubs4/4532/4532p.pdf</ref> | |||
| Due to the fact that those with limited chemistry knowledge can make GBL with easy-to-get precursors, it has become quite popular among young people in French nightclubs.<ref>{{cite web|url=https://www.residentadvisor.net/news.aspx?id=41494|title='There could be 100 comas in the year': Paris police chief reacts to rise of GBL, GHB overdoses in clubs|website=Resident Advisor|access-date=2018-04-19|archive-date=2018-04-20|archive-url=https://web.archive.org/web/20180420143837/https://www.residentadvisor.net/news.aspx?id=41494|url-status=dead}}</ref><ref>{{cite news|url=http://www.lemonde.fr/sante/article/2018/04/17/l-interdiction-de-vente-au-public-du-gbl-n-a-rien-change-a-la-consommation_5286434_1651302.html|title=Drogue : " L'interdiction de vente au public du GBL n'a rien changé à la consommation "|website=Le Monde.fr|date=17 April 2018|language=fr|access-date=2018-04-19}}</ref> | |||
| Metabolism takes place in stomach and blood plasma. The duration and onset of GBL is shorter and has a faster onset than GHB. Otherwise, effects are similar to GHB, although weight for weight it is significantly more powerful due to being absorbed faster and its higher bioavailability, meaning dosage must be lowered accordingly. If taken undiluted by mouth, GBL can cause esophageal and gastro-intestinal irritation. It is possible for oral ingestion of GBL to cause nausea and other similar problems, possibly more so than with GHB. | |||
| === |
==== Dangers ==== | ||
| If taken undiluted by mouth, GBL can cause esophageal and gastro-intestinal irritation. It is possible for oral ingestion of GBL to cause nausea and other similar problems, possibly more so than with GHB. | |||
| GHB has biphasic effects, a euphoric effect at low doses (the reason for the term ''liquid ecstasy''), and a sedative effect<ref>{{cite journal | vauthors = van Nieuwenhuijzen PS, McGregor IS | title = Sedative and hypothermic effects of gamma-hydroxybutyrate (GHB) in rats alone and in combination with other drugs: assessment using biotelemetry | journal = Drug and Alcohol Dependence | volume = 103 | issue = 3 | pages = 137–147 | date = August 2009 | pmid = 19446408 | doi = 10.1016/j.drugalcdep.2009.03.004 }}</ref> at higher doses. As a result of this sedation it can cause unconsciousness.<ref name="telegraphdeath">{{cite web | url=https://www.telegraph.co.uk/news/uknews/5895143/Coroners-Russian-roulette-warning-over-GBL-party-drug.html | title=Coroner's 'Russian roulette' warning over GBL party drug | work=The Telegraph | date=23 July 2009 | access-date=May 1, 2012 | vauthors = Edwards R }}</ref> When combined with ] the increased sedation and risk of vomiting results in a high risk of fatality. Many harm reduction organisations suggest never mixing the two drugs as a result.<ref>{{cite web | url=http://londonfriend.org.uk/get-support/drugsandalcohol/info-for-playing-safely/ghbgbl/ | title=GBL/GHB | publisher=London Friend | access-date=18 August 2014}}</ref><ref>{{cite web | url=http://www.gmfa.org.uk/ghb-and-gbl | title=GHB and GBL | publisher=GMFA | access-date=18 August 2014 | archive-date=20 June 2020 | archive-url=https://web.archive.org/web/20200620164155/https://www.gmfa.org.uk/ghb-and-gbl | url-status=dead }}</ref> | |||
| GHB (gamma hydroxybutyrate) and GBL (gamma butyrolactone) are substances which are often used as recreational drugs. GHB has two effects, at low doses it has a euphoric effect (which is why it is sometimes referred to as liquid ecstasy). At higher doses it acts like a sedative and can make the user unconscious very quickly. | |||
| There have been news reports of several deaths associated with GBL, usually in combination with alcohol or other depressants.<ref name="bbcdeath">{{cite news | vauthors = Casciani D |title=GBL drug death identified by UK doctors |url=http://news.bbc.co.uk/1/hi/uk/8428802.stm |publisher=] |date=23 December 2009 |access-date=May 1, 2012}}</ref> | |||
| ===Addictiveness=== | |||
| Gamma-Butyrolactone is often used as a ].<ref>{{cite journal | vauthors = Karila L, Novarin J, Megarbane B, Cottencin O, Dally S, Lowenstein W, Reynaud M | title = | journal = Presse Médicale | volume = 38 | issue = 10 | pages = 1526–1538 | date = October 2009 | pmid = 19762202 | doi = 10.1016/j.lpm.2009.05.017 }}</ref> | |||
| Frequent use of GHB/GBL, even when taken long-term and in moderate doses, does not appear to cause significant physical dependency in the greater majority of its users. In many people, quitting or temporarily abstaining from use of the drugs is achieved with minimal or no difficulty. However, when consumed in excessive amounts with a high frequency of dosing, physical and psychological dependence can develop.<ref>http://www.psychoactive.org.uk/GHB/addiction.htm</ref><ref>http://www.adanz.org.nz/Helpline/Subnav/Drug%20Information/ghb</ref> | |||
| ====Addictiveness and dependence==== | |||
| Frequent use of alcohol (which induces similar effects) can directly translate to physical and usually psychological addiction, where the heavy user can suffer painful and even life-threatening withdrawals. | |||
| Frequent use of GHB or GBL, even when taken long-term and in moderate doses, does not appear to cause significant physical dependency in the majority of its users. In many people, quitting or temporarily abstaining from use of the drugs is achieved with minimal or no difficulty. However, when consumed in excessive amounts with a high frequency of dosing, physical and psychological dependence can develop.<ref>{{Cite web|url=http://www.psychoactive.org.uk/GHB/addiction.htm|archiveurl=https://web.archive.org/web/20100726165005/http://www.psychoactive.org.uk/GHB/addiction.htm|url-status=dead|title=GHB addiction, GHB physical n psychological dependency levels|archivedate=July 26, 2010}}</ref> Management of GBL dependence involves considering the person's age, comorbidity and the pharmacological pathways of GBL.<ref name="pmid28186869">{{cite journal |vauthors=Santos C, Olmedo RE |title=Sedative-Hypnotic Drug Withdrawal Syndrome: Recognition And Treatment |journal=Emerg Med Pract |volume=19 |issue=3 |pages=1–20 |year=2017 |pmid=28186869 }}</ref> | |||
| GHB and GBL users can adopt a '24/7' dosing regime.<ref>{{Cite web |url=http://www.crew2000.org.uk/news/9/91/GHB-GBL-Dependancy.html |title=Crew 2000 {{!}} GHB/ GBL Dependancy [sic] {{!}} {{!}} Drugs information, advice & support, Scotland, UK<!-- Bot generated title --> |access-date=2010-08-06 |archive-date=2016-03-19 |archive-url=https://web.archive.org/web/20160319012324/http://www.crew2000.org.uk/news/9/91/ghb-gbl-dependancy.html |url-status=dead }}</ref> This is where the user has become tolerant to the effects of the drug, increasing the dosage and frequency of dosage simply to avoid withdrawal symptoms. | |||
| For those users who do report withdrawal symptoms upon quitting the use of GHB |
For those users who do report withdrawal symptoms upon quitting the use of GHB or GBL, symptoms seem to depend on the dosage and the length of time the drug was used. Light to moderate users often experience insomnia and sleep-related problems, whereas heavy, prolonged use can cause severe withdrawal symptoms similar to ] (BWS). | ||
| ===Dose=== | ====Dose==== | ||
| A milliliter of pure GBL metabolizes to |
A milliliter of pure GBL metabolizes to the equivalent 1.65 g of ], the common form, so doses are measured in the single milliliter range, either taken all at once or sipped over the course of a night. | ||
| ===Legal status |
===Legal status=== | ||
| {{update section|date=January 2020}} | |||
| '''Canada:''' GBL is a Controlled Substance under Schedule VI of the "Controlled Drugs and Substances Act" in Canada. Schedule VI of the "Controlled Drugs and Substances Act" requires vendors to collect information regarding purchases of GBL. It is not illegal for an individual to possess GBL in Canada.{{Citation needed|date=August 2009}} | |||
| '''Australia:''' GBL is not classified as a drug but as a health-endangering substance. Legislation entering into force on 1 April 2011 made it possible to handle narcotics for industrial purposes and enabled GBL and 1,4-Butanediol to be classified as controlled substances.<ref>{{Cite web|url=http://www8.austlii.edu.au/cgi-bin/viewdoc/au/legis/cth/num_act/lajladoaoma2005722/sch1.html|title=LAW AND JUSTICE LEGISLATION AMENDMENT (SERIOUS DRUG OFFENCES AND OTHER MEASURES) ACT 2005 NO. 129, 2005 - SCHEDULE 1 - Serious drug offences}}</ref> As of 2023 there are penalties for possessing, selling or driving under the influence of the substance.<ref>{{Cite web |title=GHB - Alcohol and Drug Foundation |url=https://adf.org.au/drug-facts/ghb/ |access-date=2023-07-17 |website=adf.org.au |language=en}}</ref> | |||
| '''Canada:''' GBL is a Controlled Substance under Schedule VI of the "Controlled Drugs and Substances Act" in Canada. Schedule VI of the "Controlled Drugs and Substances Act" requires vendors to collect information regarding purchases of GBL. The Act also prohibits the import and export of GBL into or out of Canada classifying it as either an indictable offense punishable with up to 10 years in prison or an offense punishable on summary conviction liable to imprisonment for up to eighteen months.<ref>{{Cite web|url=https://laws-lois.justice.gc.ca/eng/acts/C-38.8/page-3.html|title=Consolidated federal laws of Canada, Controlled Drugs and Substances Act| author = Legislative Services Branch |date=May 19, 2023|website=laws-lois.justice.gc.ca}}</ref> It is not illegal for an individual to possess GBL in Canada.{{Citation needed|date=August 2009}} | |||
| '''United Kingdom:''' GBL was classified as a Class C drug from 23 December 2009, with a prison term of up to two years for possession and 14 years for dealing, by the end of 2009.<ref>The Misuse of Drugs Act 1971 (Amendment) Order 2009 http://www.opsi.gov.uk/si/si2009/draft/ukdsi_9780111486610_en_1</ref> | |||
| '''Germany:''' GBL is not listed in the narcotics law, but its distribution is controlled. Possession is not illegal, but may be punished according to the Medicines Act, when intended to be sold for human consumption or synthesis of GHB. In recent years, an increase of GBL consumption has been observed due to the prohibition of GHB. | |||
| '''Sweden:''' GBL is not classified as a drug but as a health-endangering substance.<ref>]</ref> Although recently passed legislation to enter into force on 1 April 2011 will make it possible to handle narcotics for industrial purposes will enable GBL and 1,4-Butanediol to be classified as controlled substances.<ref>http://www.riksdagen.se/Webbnav/index.aspx?nid=3120&doktyp=betankande&bet=2010/11:SoU5</ref> | |||
| '''Hong Kong SAR:''' GBL is a dangerous drug controlled under Schedule 1 of the Dangerous Drugs Ordinance, Cap.134 (with exemption clause at Paragraph 16D). Any person who is found to have in his possession of it not in accordance with this Ordinance can be liable, on conviction upon indictment, a fine of HK$1,000,000 and to imprisonment for 7 years. | |||
| '''Australia:''' GBL is a border controlled substance and is illegal to import into Australia without a permit. The importation of a commercial quantity of a border controlled drug (over 1kg of GBL) is punishable by up to life imprisonment and/or an $825000 fine. <ref>LAW AND JUSTICE LEGISLATION AMENDMENT (SERIOUS DRUG OFFENCES AND OTHER MEASURES) ACT 2005 NO. 129, 2005 - SCHEDULE 1 http://www.austlii.edu.au/au/legis/cth/num_act/lajladoaoma2005722/sch1.html</ref> | |||
| '''Israel:''' GBL was classified as a proscribed substance from 2007.<ref>{{Cite web|url=https://www.nevo.co.il/Law_word/law01/P170_001.doc|title=section 7c of chapter B of part A of the 1st appendix of the Dangerous Drugs Act 1973|website=www.nevo.co.il}}</ref> | |||
| '''United States:''' GBL is regulated as a List 1 controlled chemical. As a GHB analog, it is treated as a controlled substance under Schedule I of the "Controlled Substances Act" if intended for human consumption.<ref></ref> | |||
| '''Netherlands''': GBL is unlike GHB not listed in the narcotics law,<ref>{{cite web|url=https://wetten.overheid.nl/BWBR0001941/2019-07-19#BijlageI|title=wetten.nl - Regeling - Opiumwet - BWBR0001941|date=19 July 2019|access-date=19 July 2019|work=]|language=nl}}</ref> but its distribution is controlled. Possession is not illegal but may be punished according to the Medicines Act, when intended to be sold for human consumption or synthesis of GHB.<ref>{{cite web|url=http://www.emerce.nl/nieuws/webwinkels-gestopt-handel-gbl|title=Webwinkels gestopt met handel in GBL|date=9 December 2013|access-date=9 December 2013|work=]|language=nl}}</ref> | |||
| '''Poland:''' In ], GBL is not classified as a drug and can be purchased in chemistry shops as a solvent. | |||
| '''People's Republic of China''': GBL was regulated as a Class III drug precursor since 7 June 2021.<ref>{{cite web|url=http://www.gov.cn/zhengce/content/2021-06/07/content_5615890.htm|title=国务院办公厅关于同意将α-苯乙酰乙酸甲酯等6种物质列入易制毒化学品品种目录的函|publisher=The State Council - The People's Republic of China|language=zh-hans|date=7 June 2021|accessdate=11 October 2021}}</ref> | |||
| '''Germany:''' GBL is not listed in the narcotics law, but its distribution is controlled. Possession is not illegal, but may be punished according to the Medicines Act, when intended to be sold for human consumption or synthesis of GHB. In recent years, an increase of GBL consumption has been observed due to the prohibition of GHB. | |||
| '''Poland:''' GBL is classified as a drug. A license is mandatory for the manufacture, processing, reworking, importing, distribution of GBL.<ref>{{Cite web|url=https://www.gif.gov.pl/pl/decyzje-i-komunikaty/komunikaty/874,KOMUNIKAT-Nr-32016-GLOWNEGO-INSPEKTORA-FARMACEUTYCZNEGO.html|title = Główny Inspektorat Farmaceutyczny}}</ref> | |||
| '''Russia''': GBL has been classified as a psychotropic substance since 22 February 2012. Its trafficking is limited, and non-licensed selling, buying or any other use is punishable by imprisonment up to 20 years. | |||
| '''Sweden:''' GBL is not classified as a drug but as a health-endangering substance. Although recently passed legislation to enter into force on 1 April 2011 will make it possible to handle narcotics for industrial purposes will enable GBL and 1,4-Butanediol to be classified as controlled substances.<ref>{{Cite web|url=http://www.riksdagen.se/Webbnav/index.aspx?nid=3120&doktyp=betankande&bet=2010/11:SoU5|title=Socialutskottets betänkande 2010/11:SoU5 - Riksdagen<!-- Bot generated title -->}}</ref> | |||
| '''United Kingdom:''' Because of their legitimate uses, regulation 4B of the 2001 regulations makes it lawful to import, export, produce, supply, offer to supply or possess GBL and ], except where a person does so knowing or believing that they will be used for the purpose of human ingestion.<ref name="HO circular">{{cite journal |title = A Change to the Misuse of Drugs Act 1971 : Control of GBL, 1,4-BD, BZP and related piperazine compounds, a further group of anabolic steroids and 2 non-steroidal agents, synthetic cannabinoid receptor agonists and oripavine | journal = Home Office Circular | volume = 21 | date = 2009 | publisher = U.K. Home Office |url = http://www.gov.uk/government/uploads/system/uploads/attachment_data/file/100526/21-2009.pdf |access-date = 2014-03-31 |archive-date = 2014-10-06 |archive-url = https://web.archive.org/web/20141006151909/https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/100526/21-2009.pdf |url-status = dead }}</ref><ref>{{cite web| title = UK Statutory Instrument 2011 No. 448 |url=http://www.legislation.gov.uk/uksi/2011/448/made?view=plain | date = 2011-02-18}}</ref> Otherwise it is a class B controlled substance.<ref>{{Cite web|url=https://www.legislation.gov.uk/uksi/2022/322/note/made|title=The Misuse of Drugs Act 1971 (Amendment) Order 2022}}</ref> | |||
| '''United States:''' GBL is regulated as a ] controlled chemical. As a GHB analog, it is also treated as a controlled substance under ] of the ] if intended for human consumption.<ref>{{Cite web|url=https://www.justice.gov/archive/ndic/pubs1/1621/index.htm|title=Information Bulletin: GHB Analogs; GBL, BD, GHV, and GVL|website=www.justice.gov}}</ref> Sales and distribution of this product for industrial use is tightly regulated and requires quantity tracing, lock and key storage and 24 hour surveillance and is limited to a very few suppliers who have appropriate DEA registrations and as of 2021 included only Ashland, BASF, and Miami Chemical. Lyondell reportedly stopped commercial sales of this product due to increasingly tight regulations and liabilities but still makes it for internal and downstream production use. To purchase this chemical requires special DEA license and end use certificate approved and a site audit by DEA. | |||
| '''Israel:''' GBL was classified as a proscribed substance from 2007, <ref>section 7c of chapter B of part A of the 1st appendix of the Dangerous Drugs Act 1973 http://www.nevo.co.il/Law_word/law01/P170_001.doc</ref>. | |||
| ==See also== | ==See also== | ||
| * ] | |||
| * ] (GHB) | |||
| * ] | |||
| * ] (1,4-B) | |||
| ==References== | ==References== | ||
| {{ |
{{reflist|2}} | ||
| ==External links== | ==External links== | ||
| * | * | ||
| * BBC News |
* {{cite news |title=The paint stripper drug that kills |publisher=BBC News |date=October 7, 2005 |url=http://news.bbc.co.uk/2/hi/uk_news/magazine/4261788.stm}} | ||
| * a NIDA Neuroscience Consortium and OSPC |
* a NIDA Neuroscience Consortium and OSPC "Cutting Edge" colloquium (27 June 2000 at the Doubletree hotel, Rockville, MD) | ||
| {{Depressants}} | |||
| {{Hypnotics}} | {{Hypnotics}} | ||
| {{GHBergics}} | {{GHBergics}} | ||
| {{GABAergics}} | {{GABAergics}} | ||
| {{Neurotoxins}} | |||
| {{DEFAULTSORT:Butyrolactone, Gamma-}} | |||
| {{DEFAULTSORT:Butyrolactone, γ-}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 08:32, 30 December 2024
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name Oxolan-2-one | |
| Other names
Dihydrofuran-2(3H)-one GBL Butyrolactone 1,4-Lactone 4-Butyrolactone 4-Hydroxybutyric acid lactone gamma-Hydroxybutyric acid lactone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.002.282 |
| IUPHAR/BPS | |
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H6O2 |
| Molar mass | 86.090 g·mol |
| Appearance | Colorless liquid |
| Odor | Weak characteristic odor, comparable to stale water, synthetic melon aroma or burnt plastic |
| Density | 1.1286 g/mL (15 °C), 1.1296 g/mL (20 °C) |
| Melting point | −43.53 °C (−46.35 °F; 229.62 K) |
| Boiling point | 204 °C (399 °F; 477 K) |
| Solubility in water | Miscible |
| Solubility | Soluble in CCl4, methanol, ethanol, acetone, benzene, ethyl ether |
| log P | −0.76 |
| Vapor pressure | 1.5 kPa (20 °C) |
| Acidity (pKa) | 4.5 |
| Refractive index (nD) | 1.435, 1.4341 (20 °C) |
| Viscosity | 1.7 cP (25 °C) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Toxic and flammable |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H302, H318, H336 |
| Precautionary statements | P264, P270, P280, P301+P312, P305+P351+P338, P403+P233, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | 98 °C (208 °F; 371 K) (closed cup) |
| Autoignition temperature |
455 °C (851 °F; 728 K) |
| Explosive limits | 3.6% v/v (lower) 16% v/v (upper) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 1540 mg/kg (oral, rat) >5640 mg/kg (dermal, rabbit) |
| LC50 (median concentration) | >2.68 mg/kg (rat, 4h) |
| Safety data sheet (SDS) | Fisher Scientific |
| Legal status |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
γ-Butyrolactone (GBL) or gamma-butyrolactone is an organic compound with the formula O=CO(CH2)3. It is a hygroscopic, colorless, water-miscible liquid with a weak characteristic odor. It is the simplest 4-carbon lactone. It is mainly used as an intermediate in the production of other chemicals, such as N-methyl-2-pyrrolidone.
In humans, GBL acts as a prodrug for gamma-hydroxybutyric acid (GHB) and is often used as a recreational drug. GHB acts as a central nervous system (CNS) depressant with effects similar to those of barbiturates.
Occurrence
GBL has been found in extracts from samples of unadulterated wines. This finding indicates that GBL is a naturally occurring component in some wines and may be present in similar products. The concentration detected was approximately 5 μg/mL and was easily observed using a simple extraction technique followed by GC/MS analysis. GBL can be found in cheese flavorings but typically results in a content of 0.0002% GBL in the final foodstuff.
Production and synthesis
γ-Butyrolactone is produced industrially by dehydrogenation of 1,4-butanediol at a temperature of 180–300 °C and atmospheric pressure in the presence of a copper catalyst.
The yield of this process is approximately 95%. The purification takes place with a liquid-gas-phase extraction.
In the laboratory, it may also be obtained via the oxidation of tetrahydrofuran (THF), for example with aqueous sodium bromate. An alternative route proceeds from GABA via a diazonium intermediate.
Reactions
As a lactone, GBL is hydrolyzed under basic conditions, for example in a sodium hydroxide solution into sodium gamma-hydroxybutyrate, the sodium salt of gamma-hydroxybutyric acid. In acidic water, a mixture of the lactone and acid forms exists in an equilibrium. These compounds then may go on to form the polymer poly(4-hydroxybutyrate) as well as the dimer 1,6-Dioxecane-2,7-dione. When treated with a non-nucleophilic base, such as lithium diisopropylamide, GBL undergoes deprotonation of the alpha carbon atom to the carbonyl. The related compound caprolactone can be used to make a polyester in this manner.
Polymerization
The ring-opening polymerization of butyrolactone gives polybutyrolactone. The resulting reverts to the monomer by thermal cracking. It is claimed that poly(GBL) is competitive with commercial biomaterial poly(4-hydroxybutyrate), or P4HB. It is further claimed that poly(GBL) is cheaper to make than P4HB, although both are bio-derived.
Uses
Gamma-Butyrolactone is used as a chemical solvent and a cleaning agent, for example in paint stripping or for cleaning graffiti. Butyrolactone is a precursor to other chemicals. Reaction with methylamine gives NMP, and with ammonia gives pyrrolidone. It is also used as a solvent in lotions and some polymers.

Butyrolactone, with its wide liquid range, chemical stability, and high dielectric constant, is used in electrolytic capacitors as the organic solvent. It is frequently mixed with a small ratio of ethylene glycol, "9:1" being common, to vary internal resistivity.
It has been used as a solvent in various laboratory experiments, e.g., the preparation of methylammonium lead halide.
GBL is used in the synthesis of DPH-362, Atiprosin, Furomazine, GET-73 , Alpertine, & McN 4612-z (page 1451 cmp. 72).
GBL is known to be used in the synthesis of 4-chlorobutyryl chloride. This in turn is used in the synthesis of the butyrophenone sidechain of many antipsychotic drugs (e.g. haloperidol).
It is also known to be employed in the synthesis of 1-bromo-4,4-bis(p-fluorophenyl)butane (the side-chain to the diphenylbutylpiperidine agents).

It was claimed that the reaction of GBL with benzene in the presence of aluminium trichloride could furnish 1-tetralone in a single step.
Another discovered GBL utility is in the synthesis of nicotine (analogs):
Pharmacology
Main article: gamma-hydroxybutyrate § PharmacologyGBL is not active in its own right; its mechanism of action stems from its identity as a prodrug of GHB.
Pharmacokinetics
GBL is rapidly converted into GHB by paraoxonase (lactonase) enzymes, found in the blood. Animals which lack these enzymes exhibit no effect from GBL. GBL is more lipophilic (fat soluble) than GHB, and so is absorbed faster and has higher bioavailability. Because of these pharmacokinetic differences, GBL tends to be more potent and faster-acting than GHB, but has a shorter duration; whereas the related compound 1,4-butanediol (1,4-B) tends to be slightly less potent and slower to take effect but longer-acting than GHB.

Nutritional supplement
Main article: Gamma-Hydroxybutyric acid § Sports and athleticsDue to its property of being a prodrug of GHB which increases sleep related growth hormone (GH) secretion, GBL was sold as a nutritional supplement after the scheduling of GHB, under the names Revivarant and Renewtrient, until they were banned by the FDA.
Recreational drug
GBL is a prodrug of GHB (naturally produced) and its recreational use comes entirely as a result of this. GBL overdose can cause irrational behavior, severe sickness, coma and death.
To bypass GHB restriction laws, home synthesis kits were introduced to transform GBL and/or 1,4-B into GHB.


GBL has a distinctive taste and odor, described as being comparable to stale water, synthetic melon aroma or burnt plastic. This differs significantly from GHB, which is described as having a decidedly "salty" taste.
Due to the fact that those with limited chemistry knowledge can make GBL with easy-to-get precursors, it has become quite popular among young people in French nightclubs.
Dangers
If taken undiluted by mouth, GBL can cause esophageal and gastro-intestinal irritation. It is possible for oral ingestion of GBL to cause nausea and other similar problems, possibly more so than with GHB.
GHB has biphasic effects, a euphoric effect at low doses (the reason for the term liquid ecstasy), and a sedative effect at higher doses. As a result of this sedation it can cause unconsciousness. When combined with alcohol the increased sedation and risk of vomiting results in a high risk of fatality. Many harm reduction organisations suggest never mixing the two drugs as a result.
There have been news reports of several deaths associated with GBL, usually in combination with alcohol or other depressants.
Gamma-Butyrolactone is often used as a date rape drug.
Addictiveness and dependence
Frequent use of GHB or GBL, even when taken long-term and in moderate doses, does not appear to cause significant physical dependency in the majority of its users. In many people, quitting or temporarily abstaining from use of the drugs is achieved with minimal or no difficulty. However, when consumed in excessive amounts with a high frequency of dosing, physical and psychological dependence can develop. Management of GBL dependence involves considering the person's age, comorbidity and the pharmacological pathways of GBL.
GHB and GBL users can adopt a '24/7' dosing regime. This is where the user has become tolerant to the effects of the drug, increasing the dosage and frequency of dosage simply to avoid withdrawal symptoms.
For those users who do report withdrawal symptoms upon quitting the use of GHB or GBL, symptoms seem to depend on the dosage and the length of time the drug was used. Light to moderate users often experience insomnia and sleep-related problems, whereas heavy, prolonged use can cause severe withdrawal symptoms similar to Benzodiazepine withdrawal syndrome (BWS).
Dose
A milliliter of pure GBL metabolizes to the equivalent 1.65 g of NaGHB, the common form, so doses are measured in the single milliliter range, either taken all at once or sipped over the course of a night.
Legal status
| This section needs to be updated. Please help update this article to reflect recent events or newly available information. (January 2020) |
Australia: GBL is not classified as a drug but as a health-endangering substance. Legislation entering into force on 1 April 2011 made it possible to handle narcotics for industrial purposes and enabled GBL and 1,4-Butanediol to be classified as controlled substances. As of 2023 there are penalties for possessing, selling or driving under the influence of the substance.
Canada: GBL is a Controlled Substance under Schedule VI of the "Controlled Drugs and Substances Act" in Canada. Schedule VI of the "Controlled Drugs and Substances Act" requires vendors to collect information regarding purchases of GBL. The Act also prohibits the import and export of GBL into or out of Canada classifying it as either an indictable offense punishable with up to 10 years in prison or an offense punishable on summary conviction liable to imprisonment for up to eighteen months. It is not illegal for an individual to possess GBL in Canada.
Germany: GBL is not listed in the narcotics law, but its distribution is controlled. Possession is not illegal, but may be punished according to the Medicines Act, when intended to be sold for human consumption or synthesis of GHB. In recent years, an increase of GBL consumption has been observed due to the prohibition of GHB.
Hong Kong SAR: GBL is a dangerous drug controlled under Schedule 1 of the Dangerous Drugs Ordinance, Cap.134 (with exemption clause at Paragraph 16D). Any person who is found to have in his possession of it not in accordance with this Ordinance can be liable, on conviction upon indictment, a fine of HK$1,000,000 and to imprisonment for 7 years.
Israel: GBL was classified as a proscribed substance from 2007.
Netherlands: GBL is unlike GHB not listed in the narcotics law, but its distribution is controlled. Possession is not illegal but may be punished according to the Medicines Act, when intended to be sold for human consumption or synthesis of GHB.
People's Republic of China: GBL was regulated as a Class III drug precursor since 7 June 2021.
Poland: GBL is classified as a drug. A license is mandatory for the manufacture, processing, reworking, importing, distribution of GBL.
Russia: GBL has been classified as a psychotropic substance since 22 February 2012. Its trafficking is limited, and non-licensed selling, buying or any other use is punishable by imprisonment up to 20 years.
Sweden: GBL is not classified as a drug but as a health-endangering substance. Although recently passed legislation to enter into force on 1 April 2011 will make it possible to handle narcotics for industrial purposes will enable GBL and 1,4-Butanediol to be classified as controlled substances.
United Kingdom: Because of their legitimate uses, regulation 4B of the 2001 regulations makes it lawful to import, export, produce, supply, offer to supply or possess GBL and 1,4-BD, except where a person does so knowing or believing that they will be used for the purpose of human ingestion. Otherwise it is a class B controlled substance.
United States: GBL is regulated as a List I controlled chemical. As a GHB analog, it is also treated as a controlled substance under Schedule I of the Controlled Substances Act if intended for human consumption. Sales and distribution of this product for industrial use is tightly regulated and requires quantity tracing, lock and key storage and 24 hour surveillance and is limited to a very few suppliers who have appropriate DEA registrations and as of 2021 included only Ashland, BASF, and Miami Chemical. Lyondell reportedly stopped commercial sales of this product due to increasingly tight regulations and liabilities but still makes it for internal and downstream production use. To purchase this chemical requires special DEA license and end use certificate approved and a site audit by DEA.
See also
References
- Merck Index, 12th edition, 1632.
- Lide DR, ed. (2009-06-03). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, FL: CRC Press. ISBN 978-1-4200-9084-0. Archived from the original on 2011-07-16. Retrieved 2011-07-18.
- "gamma-Butyrolactone". www.chemsrc.com.
- Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Schwarz W, Schossig J, Rossbacher R, Pinkos R, Höke H (2019). "Butyrolactone". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_495.pub2. ISBN 978-3527306732.
- Schep LJ, Knudsen K, Slaughter RJ, Vale JA, Mégarbane B (July 2012). "The clinical toxicology of γ-hydroxybutyrate, γ-butyrolactone and 1,4-butanediol". Clinical Toxicology. 50 (6): 458–470. doi:10.3109/15563650.2012.702218. PMID 22746383. S2CID 19697449.
- Vose J, Tighe T, Schwartz M, Buel E (September 2001). "Detection of gamma-butyrolactone (GBL) as a natural component in wine". Journal of Forensic Sciences. 46 (5): 1164–1167. doi:10.1520/JFS15116J. PMID 11569560.
- Elliott S, Burgess V (July 2005). "The presence of gamma-hydroxybutyric acid (GHB) and gamma-butyrolactone (GBL) in alcoholic and non-alcoholic beverages". Forensic Science International. 151 (2–3): 289–292. doi:10.1016/j.forsciint.2005.02.014. PMID 15939164.
- ^ "A Change to the Misuse of Drugs Act 1971 : Control of GBL, 1,4-BD, BZP and related piperazine compounds, a further group of anabolic steroids and 2 non-steroidal agents, synthetic cannabinoid receptor agonists and oripavine" (PDF). Home Office Circular. 21. U.K. Home Office. 2009. Archived from the original (PDF) on 2014-10-06. Retrieved 2014-03-31.
- Metsger L, Bittner S (March 2000). "Autocatalytic Oxidation of Ethers with Sodium Bromate". Tetrahedron. 56 (13): 1905–1910. doi:10.1016/S0040-4020(00)00098-3.
- "Sandmeyer Reaction of GABA to GBL/GHB". Retrieved 2018-06-14.
- ^ Micu A (December 12, 2015). "New, fully recyclable and biodegradable plastic could change the world". ZME Science. Retrieved 2015-12-13.
- Hong M, Chen EY (January 2016). "Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of γ-butyrolactone". Nature Chemistry. 8 (1): 42–49. doi:10.1038/nchem.2391. PMID 26673263.
- Hong M, Chen EY (March 2016). "Towards Truly Sustainable Polymers: A Metal-Free Recyclable Polyester from Biorenewable Non-Strained γ-Butyrolactone". Angewandte Chemie. 55 (13): 4188–4193. doi:10.1002/anie.201601092. PMID 26934184.
- "Gamma-Gamma-Butyrolactone:an industrial solvent". Chemical Book. Retrieved 21 June 2024.
- "Insider tell you why the GBL Cleaner is the best solution for these nasty dirties and stains". Medium. 16 December 2014. Retrieved 21 June 2024.
- Niu T, Lu J, Munir R, Li J, Barrit D, Zhang X, et al. (2018). "Stable High-Performance Perovskite Solar Cells via Grain Boundary Passivation". Advanced Materials. 30 (16). doi:10.1002/adma.201706576. PMID 29527750.
- Anton Ebnother, Ernst Jucker, Erwin Rissi, U.S. patent 3,531,480 (1970 to Sandoz Ag).
- EP0932597 idem Roberto Cacciaglia, et al. WO1998006690 (Laboratorio Farmaceutico CT SRL).
- Roberto Cacciaglia & Massimo Ferrari, WO2009062949 (Laboratorio Farmaceutico CT SRL).
- Sydney Archer, US3562278 & US3639414 (1971 to Sterling Drug Inc).
- Maryanoff BE, McComsey DF, Gardocki JF, Shank RP, Costanzo MJ, Nortey SO, et al. (August 1987). "Pyrroloisoquinoline antidepressants. 2. In-depth exploration of structure-activity relationships". Journal of Medicinal Chemistry. 30 (8): 1433–54. doi:10.1021/jm00391a028. PMID 3039136.
- CN101445447A
- Junying Yuan, et al. WO2011143444 ().
- "A-TETRALONE". Organic Syntheses. 35: 95. 1955. doi:10.15227/orgsyn.035.0095.
- Huang K, Ortiz-Marciales M, De Jesús M, Stepanenko V (2009). "A new and efficient approach to the synthesis of nicotine and anabasine analogues". Journal of Heterocyclic Chemistry. 46 (6): 1252–1258. doi:10.1002/jhet.233. PMC 2811585. PMID 20161612.
- Snead OC (February 1991). "The gamma-hydroxybutyrate model of absence seizures: correlation of regional brain levels of gamma-hydroxybutyric acid and gamma-butyrolactone with spike wave discharges". Neuropharmacology. 30 (2): 161–167. doi:10.1016/0028-3908(91)90199-l. PMID 2030821.
- ^ Kobilinsky L (2011-11-29). Forensic Chemistry Handbook. John Wiley & Sons. p. 386. ISBN 978-0-471-73954-8.
- Teiber JF, Draganov DI, La Du BN (September 2003). "Lactonase and lactonizing activities of human serum paraoxonase (PON1) and rabbit serum PON3". Biochemical Pharmacology. 66 (6): 887–896. doi:10.1016/S0006-2952(03)00401-5. PMID 12963475.
- "Gamma-butyrolactone (GBL) Pre-Review Report" (PDF). 4 June 2012.
- Van Cauter E, Plat L, Scharf MB, Leproult R, Cespedes S, L'Hermite-Balériaux M, et al. (August 1997). "Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men". The Journal of Clinical Investigation. 100 (3): 745–753. doi:10.1172/JCI119587. PMC 508244. PMID 9239423.
- "Erowid GHB vault: FDA Warning about Gamma Butyrlactone". Erowid. 1998-11-21. Retrieved 2013-10-10.
- Meyer J, Quenzer LF (2005). Psychopharmacology: Drugs, the Brain and Behavior. Sinauer. p. 370. ISBN 978-0-87893-534-5.
- "USDOJ: U.S. Department of Justice Archive National Drug Intelligence Center" (PDF). Usdoj.gov. 2012-06-15. Retrieved 2014-01-22.
- Galloway GP, Frederick-Osborne SL, Seymour R, Contini SE, Smith DE (April 2000). "Abuse and therapeutic potential of gamma-hydroxybutyric acid". Alcohol. 20 (3): 263–269. doi:10.1016/S0741-8329(99)00090-7. PMID 10869868.
- "'There could be 100 comas in the year': Paris police chief reacts to rise of GBL, GHB overdoses in clubs". Resident Advisor. Archived from the original on 2018-04-20. Retrieved 2018-04-19.
- "Drogue : " L'interdiction de vente au public du GBL n'a rien changé à la consommation "". Le Monde.fr (in French). 17 April 2018. Retrieved 2018-04-19.
- van Nieuwenhuijzen PS, McGregor IS (August 2009). "Sedative and hypothermic effects of gamma-hydroxybutyrate (GHB) in rats alone and in combination with other drugs: assessment using biotelemetry". Drug and Alcohol Dependence. 103 (3): 137–147. doi:10.1016/j.drugalcdep.2009.03.004. PMID 19446408.
- Edwards R (23 July 2009). "Coroner's 'Russian roulette' warning over GBL party drug". The Telegraph. Retrieved May 1, 2012.
- "GBL/GHB". London Friend. Retrieved 18 August 2014.
- "GHB and GBL". GMFA. Archived from the original on 20 June 2020. Retrieved 18 August 2014.
- Casciani D (23 December 2009). "GBL drug death identified by UK doctors". BBC News. Retrieved May 1, 2012.
- Karila L, Novarin J, Megarbane B, Cottencin O, Dally S, Lowenstein W, et al. (October 2009). "". Presse Médicale. 38 (10): 1526–1538. doi:10.1016/j.lpm.2009.05.017. PMID 19762202.
- "GHB addiction, GHB physical n psychological dependency levels". Archived from the original on July 26, 2010.
- Santos C, Olmedo RE (2017). "Sedative-Hypnotic Drug Withdrawal Syndrome: Recognition And Treatment". Emerg Med Pract. 19 (3): 1–20. PMID 28186869.
- "Crew 2000 | GHB/ GBL Dependancy [sic] | | Drugs information, advice & support, Scotland, UK". Archived from the original on 2016-03-19. Retrieved 2010-08-06.
- "LAW AND JUSTICE LEGISLATION AMENDMENT (SERIOUS DRUG OFFENCES AND OTHER MEASURES) ACT 2005 NO. 129, 2005 - SCHEDULE 1 - Serious drug offences".
- "GHB - Alcohol and Drug Foundation". adf.org.au. Retrieved 2023-07-17.
- Legislative Services Branch (May 19, 2023). "Consolidated federal laws of Canada, Controlled Drugs and Substances Act". laws-lois.justice.gc.ca.
- "section 7c of chapter B of part A of the 1st appendix of the Dangerous Drugs Act 1973". www.nevo.co.il.
- "wetten.nl - Regeling - Opiumwet - BWBR0001941". Wetten.nl (in Dutch). 19 July 2019. Retrieved 19 July 2019.
- "Webwinkels gestopt met handel in GBL". Emerce (in Dutch). 9 December 2013. Retrieved 9 December 2013.
- "国务院办公厅关于同意将α-苯乙酰乙酸甲酯等6种物质列入易制毒化学品品种目录的函" (in Simplified Chinese). The State Council - The People's Republic of China. 7 June 2021. Retrieved 11 October 2021.
- "Główny Inspektorat Farmaceutyczny".
- "Socialutskottets betänkande 2010/11:SoU5 - Riksdagen".
- "UK Statutory Instrument 2011 No. 448". 2011-02-18.
- "The Misuse of Drugs Act 1971 (Amendment) Order 2022".
- "Information Bulletin: GHB Analogs; GBL, BD, GHV, and GVL". www.justice.gov.
External links
- Erowid on GBL
- "The paint stripper drug that kills". BBC News. October 7, 2005.
- "All About GHB," a NIDA Neuroscience Consortium and OSPC "Cutting Edge" colloquium (27 June 2000 at the Doubletree hotel, Rockville, MD)
| GHB receptor modulators | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor (ligands) |
| ||||||||||
| Transporter (blockers) |
| ||||||||||
| Enzyme (inhibitors) |
| ||||||||||
| GABA receptor modulators | |||||
|---|---|---|---|---|---|
| Ionotropic |
| ||||
| Metabotropic |
| ||||