| Revision as of 21:49, 26 August 2014 edit67.148.215.250 (talk) →Sources← Previous edit | Latest revision as of 11:25, 8 January 2025 edit undo218.50.18.130 (talk) Reference: https://www.health.kr/searchDrug/result_drug.asp?drug_cd=A11AKP08G4675 Theobromine has been approved in South Korea as a prescription drug since 2008-05-28 and is being actively prescribed by doctors as of 2025-01-07 (from personal experience). It is classified as an antitussive. Translation: "A medication that relaxes and dilates bronchial smooth muscle to relieve symptoms of dyspnea caused by airway obstructive disorders." | ||
| (372 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Bitter alkaloid of the cacao plant}} | |||
| {{cs1 config|name-list-style=vanc|display-authors=6}} | |||
| {{Distinguish|bromine}} | {{Distinguish|bromine}} | ||
| {{Drugbox| Verifiedfields = changed | |||
| | Watchedfields = changed | |||
| | verifiedrevid = 419893781 | |||
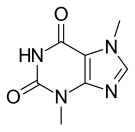
| | IUPAC_name = 3,7-dimethyl-1''H''-purine-2,6-dione | |||
| | image = Theobromin - Theobromine.svg | |||
| | image2 = Theobromine3d.png | |||
| {{Infobox drug | |||
| <!--Clinical data--> | |||
| | Watchedfields = changed | |||
| | legal_status = Uncontrolled substance | |||
| | verifiedrevid = 622941253 | |||
| | IUPAC_name = 3,7-dimethyl-1''H''-purine-2,6-dione | |||
| | image = Theobromine.svg | |||
| | width = 135 | |||
| | image2 = Theobromine 3D ball.png | |||
| <!--Clinical data-->| legal_status = In general: Unscheduled. | |||
| | dependency_liability = None | |||
| | addiction_liability = | |||
| | routes_of_administration = ] | | routes_of_administration = ] | ||
| <!--Pharmacokinetic data--> | <!--Pharmacokinetic data-->| metabolism = ] ] and ] | ||
| | elimination_half-life = 6–8 hours<ref name="halflife1">{{cite journal | vauthors = Drouillard DD, Vesell ES, Dvorchik BH | title = Studies on theobromine disposition in normal subjects. Alterations induced by dietary abstention from or exposure to methylxanthines | journal = Clinical Pharmacology and Therapeutics | volume = 23 | issue = 3 | pages = 296–302 | date = March 1978 | pmid = 627135 | doi = 10.1002/cpt1978233296 | s2cid = 10519385 }}</ref><ref name="halflife2">{{cite journal | vauthors = Lelo A, Birkett DJ, Robson RA, Miners JO | title = Comparative pharmacokinetics of caffeine and its primary demethylated metabolites paraxanthine, theobromine and theophylline in man | journal = British Journal of Clinical Pharmacology | volume = 22 | issue = 2 | pages = 177–182 | date = August 1986 | pmid = 3756065 | pmc = 1401099 | doi = 10.1111/j.1365-2125.1986.tb05246.x }}</ref> | |||
| | metabolism = ] ] and ] | |||
| | excretion = ] (10% unchanged, rest as metabolites) | |||
| | elimination_half-life = 7.1±0.7 hours | |||
| | excretion = ] (10% unchanged, rest as metabolites) | |||
| <!--Identifiers--> | <!--Identifiers-->| CAS_number_Ref = {{cascite|correct|CAS}} | ||
| | CAS_number = 83-67-0 | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | ATC_prefix = C03 | |||
| | CAS_number_Ref = {{cascite|correct|??}} | |||
| | ATC_suffix = BD01 | |||
| | CAS_number = 83-67-0 | |||
| | ATC_supplemental = {{ATC|R03|DA07}} | |||
| | ATC_prefix = C03 | |||
| | PubChem = 5429 | |||
| | ATC_suffix = BD01 | |||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | ATC_supplemental = {{ATC|R03|DA07}} | |||
| | DrugBank = DB01412 | |||
| | PubChem = 5429 | |||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 5236 | |||
| | DrugBank = DB01412 | |||
| | |
| UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = OBD445WZ5P | |||
| | ChemSpiderID = 5236 | |||
| | |
| KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = C07480 | |||
| | UNII = OBD445WZ5P | |||
| | |
| ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | ChEBI = 28946 | |||
| | KEGG = C07480 | |||
| | |
| ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | ChEMBL = 1114 | |||
| | ChEBI = 28946 | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | ChEMBL = 1114 | |||
| <!--Chemical data--> | <!--Chemical data-->| C = 7 | ||
| | H = 8 | |||
| | C=7 | H=8 | N=4 | O=2<ref name="dictbiochem1943"/> | |||
| | N = 4 | |||
| | molecular_weight = 180.164 g/mol | |||
| | O = 2 | |||
| | smiles = Cn1cnc2c1c(=O)c(=O)n2C | |||
| | chemical_formula_ref = | |||
| | InChI = 1/C7H8N4O2/c1-10-3-8-5-4(10)6(12)9-7(13)11(5)2/h3H,1-2H3,(H,9,12,13)/f/h9H | |||
| | smiles = Cn1cnc2c1c(=O)c(=O)n2C | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI = 1S/C7H8N4O2/c1-10-3-8-5-4(10)6(12)9-7(13)11(5)2/h3H,1-2H3,(H,9,12,13) | |||
| | StdInChI = 1S/C7H8N4O2/c1-10-3-8-5-4(10)6(12)9-7(13)11(5)2/h3H,1-2H3,(H,9,12,13) | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey = YAPQBXQYLJRXSA-UHFFFAOYSA-N | |||
| | StdInChIKey = YAPQBXQYLJRXSA-UHFFFAOYSA-N | |||
| | synonyms = xantheose<br />diurobromine<br />3,7-dimethylxanthine | |||
| | synonyms = xantheose<br />diurobromine<br />3,7-dimethylxanthine<br />3,7-dihydro-3,7-dimethyl-1''H''-purine-2,6-dione | |||
| }} | }} | ||
| '''Theobromine''', formerly known as '''xantheose''',<ref name="dictbiochem1943"/> is a bitter ] of the ], with the chemical formula ]]]]. It is found in ], as well as in a number of other foods, including the leaves of the ] plant, and the ] (or cola) nut. It is classified as a ] ],<ref name="envbeh-p200">{{cite book | title=Environment and Behavior | last = Baer | first = Donald M. | author2 = Elsie M. Pinkston | year=1997 | publisher=Westview Press | page=200 }}</ref> which also includes the similar compounds ] and ].<ref name="dictbiochem1943"/> The compounds differ in their degree of methylation. | |||
| {{Chembox | |||
| Despite its name, the compound contains no ]—''theobromine'' is derived from '']'', the name of the ] of the cacao tree, (which itself is made up of the ] roots ''theo'' ("]") and ''broma'' ("food"), meaning "food of the gods")<ref name="worldofcaffeine">{{cite book | last = Bennett | first = Alan Weinberg | author2 = Bonnie K. Bealer | title = The World of Caffeine: The Science and Culture of the World's Most Popular Drug | publisher = ], New York | year = 2002 | isbn = 0-415-92723-4 }} (note: the book incorrectly states that the name "theobroma" is derived from Latin)</ref> with the suffix ''-ine'' given to alkaloids and other ] nitrogen-containing compounds.<ref name="dict-ine">"-ine."{{cite book | title = The American Heritage Dictionary of the English Language, Fourth Edition | publisher = ] | year = 2004 | url = http://dictionary.reference.com/browse/-ine | isbn = 0-395-71146-0 }}</ref> | |||
| |container_only = yes | |||
| | ImageFile = | |||
| | ImageSize = | |||
| | ImageAlt = | |||
| | IUPACName = | |||
| | OtherNames = | |||
| |Section1={{Chembox Identifiers | |||
| | CASNo = | |||
| | PubChem = | |||
| | SMILES = }} | |||
| |Section2={{Chembox Properties | |||
| | Formula = | |||
| | MolarMass = | |||
| | Appearance = white solid | |||
| | Density = 1.524 g/cm<sup>3</sup><ref name=Acta/> | |||
| | MeltingPtC = 351 | |||
| | BoilingPt = | |||
| | Solubility = 330 mg/L)}} | |||
| |Section3={{Chembox Hazards | |||
| | MainHazards = | |||
| | FlashPt = | |||
| | AutoignitionPt = }} | |||
| }} | |||
| '''Theobromine''', also known as '''xantheose''', is the principal ] of '']'' (cacao plant).<ref name="pubchem">{{cite web |title=Theobromine |url=https://pubchem.ncbi.nlm.nih.gov/compound/5429 |publisher=PubChem, US National Library of Medicine |access-date=3 September 2022 |date=27 August 2022}}</ref> Theobromine is slightly water-] (330 mg/L) with a bitter taste.<ref name=Hbk>{{cite book | vauthors = Smit HJ | chapter = Theobromine and the Pharmacology of Cocoa | title = Methylxanthines | series = Handbook of Experimental Pharmacology | volume = 200 | issue = <!-- none --> | pages = 201–234 | year = 2011 | pmid = 20859797 | doi = 10.1007/978-3-642-13443-2_7 | isbn = 978-3-642-13442-5 }}</ref> In industry, theobromine is used as an ] and precursor to some ].<ref name=pubchem/> It is found in ], as well as in a number of other foods, including ] ('']''), some American ] (] and ]) and the ]. It is a white or colourless solid, but commercial samples can appear yellowish.<ref name=Hbk/> | |||
| ==Structure== | |||
| Theobromine is a slightly water-] (330 mg/L<ref>{{ChemID|83-67-0}}</ref>), ], bitter powder. Theobromine is white or colourless, but commercial samples can be yellowish.<ref name="theobromine-chemprops">{{cite web | url=http://dictionary.reference.com/search?q=theobromine&db=* | title=theobromine | publisher=Dictionary.com | accessdate=2007-02-22 }} For convenience, the direct source of the three definitions used has been cited.</ref> It has a similar, but lesser, effect to ] in the human nervous system, making it a lesser ]. Theobromine is an ] of theophylline, as well as ]. Theobromine is categorized as a ] xanthine.<ref name="omd-theobromine">{{cite web | url=http://cancerweb.ncl.ac.uk/cgi-bin/omd?query=theobromine&action=Search+OMD | title=Theobromine | publisher=On-Line Medical Dictionary | accessdate=2007-02-23 }}</ref> | |||
| Theobromine is a flat molecule,<ref name=Acta>{{cite journal |doi=10.1107/S0108270198009469|title=Methylxanthines. II. Anhydrous Theobromine |year=1998 | vauthors = Ford KA, Ebisuzaki Y, Boyle PD |journal=Acta Crystallographica Section C Crystal Structure Communications |volume=54 |issue=12 |pages=1980–1983 |bibcode=1998AcCrC..54.1980F }}</ref> a derivative of ] and an isomer of theophylline.<ref>{{cite web |title=Theophylline |url=https://pubchem.ncbi.nlm.nih.gov/compound/2153 |publisher=PubChem, US National Library of Medicine |access-date=2 September 2023 |date=26 August 2023}}</ref> It is also classified as a ] ].<ref name=Hbk/><ref name="envbeh-p200">{{cite book | title=Environment and Behavior | vauthors = Baer DM, Pinkston EM | year=1997 | publisher=Westview Press | page= | isbn=978-0813331591 | url=https://archive.org/details/environmentbehav0000unse/page/200 }}</ref> Related compounds include ], ], ], and ], each of which differ in the number or placement of the methyl groups.<ref name=Hbk/> | |||
| ==History== | |||
| Theobromine was first discovered in 1841<ref>. Books.google.ru. Retrieved on 2009-11-08.</ref> in cacao beans by Russian chemist Alexander Voskresensky.<ref>{{cite journal | author = Woskresensky A | year = 1842 | title = Über das Theobromin | url = http://books.google.com/books?id=ZE09AAAAcAAJ&pg=PA125#v=onepage&q&f=false | journal = Liebigs Annalen der Chemie und Pharmacie | volume = 41 | issue = | pages = 125–127 | doi = 10.1002/jlac.18420410117 }}</ref> Theobromine was first synthesized from ] by ].<ref name="historicalchemistry">{{cite book | title=Essays in Historical Chemistry | author=Thomas Edward Thorpe | year=1902 | publisher=The MacMillan Company }}</ref><ref>{{cite journal | author = Fischer Emil | year = 1882 | title = Umwandlung des Xanthin in Theobromin und Caffein", ''Berichte der deutsche chemischen Gesellschaft'', vol. 15, no. 1, pages 453-456. See also: Fischer, E. (1882) "Über Caffein, Theobromin, Xanthin und Guanin | url = | journal = Justus Liebigs Annalen der Chemie | volume = 215 | issue = 3| pages = 253–320 | doi = 10.1002/jlac.18822150302 }}</ref> | |||
| Theobromine was first discovered in 1841<ref>{{cite book| vauthors = von Bibra E, Ott J |title=Plant Intoxicants: A Classic Text on the Use of Mind-Altering Plants|url=https://books.google.com/books?id=EWqhC4djXSQC&pg=PA67|date=1995|publisher=Inner Traditions / Bear & Co|isbn=978-0-89281-498-5|pages=67–|access-date=2015-12-12|archive-date=2019-09-18|archive-url=https://web.archive.org/web/20190918203121/https://books.google.com/books?id=EWqhC4djXSQC&pg=PA67|url-status=live}}</ref> in cacao beans by the chemist ].<ref>{{cite journal | vauthors = Woskresensky A | year = 1842 | title = Über das Theobromin | url = https://books.google.com/books?id=ZE09AAAAcAAJ&pg=PA125 | journal = Liebigs Annalen der Chemie und Pharmacie | volume = 41 | pages = 125–127 | doi = 10.1002/jlac.18420410117 | access-date = 2015-12-12 | archive-date = 2016-06-10 | archive-url = https://web.archive.org/web/20160610112332/https://books.google.com/books?id=ZE09AAAAcAAJ&pg=PA125 | url-status = live }}</ref> Synthesis of theobromine from ] was first reported in 1882 by ].<ref name="historicalchemistry">{{cite book | title=Essays in Historical Chemistry | url=https://archive.org/details/b31350975_0002 | vauthors = Thorpe TE | year=1902 | publisher=The MacMillan Company }}</ref><ref>{{cite journal | vauthors = Fischer, Emil | year = 1882 | title = Umwandlung des Xanthin in Theobromin und Caffein | journal = Berichte der Deutschen Chemischen Gesellschaft | volume = 15 | issue = 1 | pages = 453–456 | doi = 10.1002/cber.18820150194 | url = https://zenodo.org/record/1425264 | access-date = 2019-09-09 | archive-date = 2019-05-05 | archive-url = https://web.archive.org/web/20190505192210/https://zenodo.org/record/1425264/files/article.pdf | url-status = live }}</ref><ref>{{ cite journal | vauthors = Fischer E |year=1882| title = Über Caffein, Theobromin, Xanthin und Guanin | url = https://zenodo.org/record/1427381| journal = Justus Liebigs Annalen der Chemie | volume = 215 | issue = 3| pages = 253–320 | doi = 10.1002/jlac.18822150302 }}</ref> | |||
| ==Etymology== | |||
| ''Theobromine'' is derived from '']'', the name of the ] of the cacao tree, with the suffix ''-ine'' given to alkaloids and other ] nitrogen-containing compounds.<ref name="dict-ine">{{cite book | chapter = -ine | title = The American Heritage Dictionary of the English Language, Fourth Edition | publisher = ] | year = 2004 | chapter-url = http://dictionary.reference.com/browse/-ine | isbn = 978-0-395-71146-0 | access-date = 2007-02-23 | archive-date = 2016-03-03 | archive-url = https://web.archive.org/web/20160303171459/http://dictionary.reference.com/browse/-ine | url-status = live }}</ref> That name in turn is made up of the ] roots ''theo'' ("]") and ''broma'' ("food"), meaning "food of the gods".<ref name="worldofcaffeine">{{cite book | vauthors = Bennett AW, Bealer BK | title = The World of Caffeine: The Science and Culture of the World's Most Popular Drug | publisher = ], New York | year = 2002 | isbn = 978-0-415-92723-9 | url = https://archive.org/details/worldofcaffeines00benn }} (note: the book incorrectly states that the name "theobroma" is derived from Latin)</ref> | |||
| Despite its name, the compound contains no ], which is based on Greek ''bromos'' ("stench"). | |||
| ==Sources== | ==Sources== | ||
| ], which is a natural source of theobromine.]] | ], which is a natural source of theobromine.]] | ||
| Theobromine is the primary alkaloid found in ] and ]. Cocoa powder can vary in the amount of theobromine, from 2%<ref name="Hershey_theobromine">{{cite web |url= http://www.hersheys.com/nutrition/theobromine.asp |title= Theobromine content of Hershey's confectionery products |publisher= The Hershey Company |accessdate=2008-04-07}}</ref> theobromine to at least 10%. There are usually higher concentrations in dark than in milk chocolate.<ref name="AmerMed_cocoa">{{cite web |url= http://www.amermed.com/cocoa.htm |title= AmerMed cocoa extract with 10% theobromine |publisher= AmerMed |accessdate=2008-04-13}}</ref> Theobromine can also be found in small amounts in the ] (1.0–2.5%), the ] berry, ] (yerba mate), and the ].<ref name="culthistplants">{{cite book | author=Sir Ghillean Prance, Mark Nesbitt | title=The Cultural History of Plants | publisher=Routledge | year=2004 | location=New York | pages=137, 175, 178–180 | isbn = 0-415-92746-3}}</ref> 1 oz of milk chocolate contains approximately 60 mg of theobromine<ref name="USDA db milk chocolate entry">{{cite web |url=http://ndb.nal.usda.gov/ndb/foods/show/5924?qlookup=milk+chocolate&offset=&format=Full#id-1 |title=USDA Nutrient database, entries for milk chocolate |accessdate=2012-12-29}}</ref> but 1 oz of dark chocolate contains about 200 mg.<ref name="USDA db dark chocolate entry">{{cite web |url=http://ndb.nal.usda.gov/ |title=USDA Nutrient database, entries for dark chocolate |accessdate=2012-11-07}}</ref> Cocoa beans naturally contain approximately 1% theobromine.{{http://www.ncbi.nlm.nih.gov/pubmed/1586288}} | |||
| Theobromine is the primary alkaloid found in ] and ]. ] only contains trace amounts of theobromine. There are usually higher concentrations in dark than in milk chocolate.<ref name="AmerMed_cocoa">{{cite web |url= http://www.amermed.com/cocoa.htm |title= AmerMed cocoa extract with 10% theobromine |publisher= AmerMed |access-date= 2008-04-13 |archive-date= 2015-08-11 |archive-url= https://web.archive.org/web/20150811092221/http://amermed.com/cocoa.htm |url-status= dead }}</ref> | |||
| There are approximately {{Convert|60|mg|abbr = off|0}} of theobromine in {{Convert|1|oz|order = flip}} of milk chocolate,<ref name="USDA db milk chocolate entry">{{cite web |url=http://ndb.nal.usda.gov/ndb/foods/show/5924?qlookup=milk+chocolate&offset=&format=Full#id-1 |title=USDA Nutrient database, entries for milk chocolate |access-date=2012-12-29 |archive-date=2017-07-08 |archive-url=https://web.archive.org/web/20170708121554/https://ndb.nal.usda.gov/ndb/foods/show/5924?qlookup=milk+chocolate&offset=&format=Full#id-1 |url-status=dead }}</ref> while the same amount of dark chocolate contains about {{Convert|200|mg|abbr = off|0}}.<ref name="USDA db dark chocolate entry">{{cite web |url=https://fdc.nal.usda.gov |title=USDA Nutrient database, entries for dark chocolate |access-date=2012-11-07 }}</ref> Cocoa beans naturally contain approximately 1% theobromine.<ref>{{cite journal | vauthors = Kuribara H, Tadokoro S | title = Behavioral effects of cocoa and its main active compound theobromine: evaluation by ambulatory activity and discrete avoidance in mice | journal = Arukoru Kenkyu to Yakubutsu Izon = Japanese Journal of Alcohol Studies & Drug Dependence | volume = 27 | issue = 2 | pages = 168–179 | date = April 1992 | pmid = 1586288 }}</ref> | |||
| * '']'' | |||
| * '']'' | |||
| * '']'' | |||
| * '']'' | |||
| * '']'' | |||
| * '']'' | |||
| * '']'' | |||
| * '']'' | |||
| Plant species and components with substantial amounts of theobromine are:<ref name="arsgrin-theobromine">{{cite web| url=https://phytochem.nal.usda.gov/phytochem/chemicals/show/17073?et=| title=Theobromine content in plant sources| publisher=Dr. Duke's Phytochemical and Ethnobotanical Databases, ]| date=6 February 2019| access-date=9 March 2019| archive-date=8 May 2019| archive-url=https://web.archive.org/web/20190508200500/https://phytochem.nal.usda.gov/phytochem/chemicals/show/17073?et=| url-status=dead}}</ref><ref name=":4">{{cite journal |vauthors=Crown PL, Emerson TE, Gu J, Hurst WJ, Pauketat TR, Ward T |date=August 2012 |title=Ritual Black Drink consumption at Cahokia |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=109 |issue=35 |pages=13944–9 |doi=10.1073/pnas.1208404109 |pmc=3435207 |pmid=22869743 |doi-access=free}}</ref> | |||
| The mean theobromine concentrations in cocoa and ] products are:<ref name=Craig1984> | |||
| {{cite journal | |||
| * '']'' – seed and ] | |||
| | author = Craig, Winston J. | |||
| * '']'' – seed coat | |||
| | author2 = Nguyen, Thuy T. | |||
| * '']'' – leaf | |||
| | year = 1984 | |||
| * ''] – leaf'' | |||
| | title = Caffeine and theobromine levels in cocoa and carob products | |||
| * ''] – leaf'' | |||
| | journal = ] | |||
| * '']'' – leaf | |||
| | volume = 49 | |||
| | issue = 1 | |||
| Theobromine can also be found in trace amounts in the ], the ] berry, ] (]),<ref>{{cite journal | vauthors = Schuster J, Mitchell ES | title = More than just caffeine: psychopharmacology of methylxanthine interactions with plant-derived phytochemicals | journal = Progress in Neuro-Psychopharmacology & Biological Psychiatry | volume = 89 | pages = 263–274 | date = March 2019 | pmid = 30213684 | doi = 10.1016/j.pnpbp.2018.09.005 | s2cid = 52274913 | doi-access = free }}</ref> and the ].<ref name="culthistplants">{{cite book | vauthors = Prance G, Nesbitt M | title=The Cultural History of Plants | publisher=Routledge | year=2004 | location=New York | pages=137, 175, 178–180 | isbn = 978-0-415-92746-8}}</ref> | |||
| | pages = 302–303 | |||
| | doi = 10.1111/j.1365-2621.1984.tb13737.x | |||
| The mean theobromine concentrations in cocoa and ] products are:<ref>{{cite web |title=FoodData Central |url=https://fdc.nal.usda.gov/fdc-app.html#/food-details/169593/nutrients |website=fdc.nal.usda.gov}}</ref><ref name=Craig1984>{{cite journal| vauthors = Craig WJ, Nguyen TT | year = 1984| title = Caffeine and theobromine levels in cocoa and carob products| journal = ]| volume = 49| issue = 1| pages = 302–303| doi = 10.1111/j.1365-2621.1984.tb13737.x| quote = Mean theobromine and caffeine levels respectively, were 0.695 mg/g and 0.071 mg/g in cocoa cereals; 1.47 mg/g and 0.152 mg/g in chocolate bakery products; 1.95 mg/g and 0.138 mg/g in chocolate toppings; 2.66 mg/g and 0.208 mg/g in cocoa beverages; 0.621 mg/g and 0.032 mg/g in chocolate ice creams; 0.226 mg/g and 0.011 mg/g in chocolate milks; 74.8 mg/serving and 6.5 mg/serving in chocolate puddings.... Theobromine and caffeine levels in carob products ranged from 0–0.504 mg/g and 0-0.067 mg/g, respectively.}}</ref> | |||
| | url = http://www.blackwell-synergy.com/doi/abs/10.1111/j.1365-2621.1984.tb13737.x | |||
| | accessdate = 2008-04-20 | |||
| | quote = Mean theobromine and caffeine levels respectively, were 0.695 mg/g and 0.071 mg/g in cocoa cereals; 1.47 mg/g and 0.152 mg/g in chocolate bakery products; 1.95 mg/g and 0.138 mg/g in chocolate toppings; 2.66 mg/g and 0.208 mg/g in cocoa beverages; 0.621 mg/g and 0.032 mg/g in chocolate ice creams; 0.226 mg/g and 0.011 mg/g in chocolate milks; 74.8 mg/serving and 6.5 mg/serving in chocolate puddings.... Theobromine and caffeine levels in carob products ranged from 0-0.504 mg/g and 0-0.067 mg/g, respectively. | |||
| }}</ref> | |||
| {| class="wikitable" | {| class="wikitable" | ||
| |+ | |+ | ||
| ! Item | ! Item | ||
| ! Mean theobromine |
! Mean theobromine per 100 g | ||
| |- | |- | ||
| | Cocoa | | ] | ||
| | |
| 2060 mg<!--To verify this claim, see Table 1 in the reference--> | ||
| |- | |- | ||
| | Cocoa |
| ] | ||
| | 266 mg | |||
| | 0.695 | |||
| |- | |||
| | Chocolate toppings | |||
| | 195 mg | |||
| |- | |- | ||
| | Chocolate bakery products | | Chocolate bakery products | ||
| | 147 mg | |||
| | 1.47 | |||
| |- | |- | ||
| | Cocoa cereals | |||
| | Chocolate toppings | |||
| | |
| 69.5 mg | ||
| |- | |- | ||
| | ]s | |||
| | Cocoa beverages | |||
| | |
| 62.1 mg | ||
| |- | |- | ||
| | Chocolate |
| ]s | ||
| | |
| 22.6 mg | ||
| |- | |- | ||
| | ] products | |||
| | Chocolate milks | |||
| | 0.00–50.4 mg | |||
| | 0.226 | |||
| |- | |||
| | Carob products | |||
| | 0.000–0.504 | |||
| |} | |} | ||
| ===Biosynthesis=== | ===Biosynthesis=== | ||
| Theobromine is a purine alkaloid derived from ], a ]. Cleavage of the ribose and N-methylation yields 7-methylxanthosine. 7-Methylxanthosine in turn is the precursor to theobromine, which in turn is the precursor to ].<ref>Ashihara, |
Theobromine is a purine alkaloid derived from ], a ]. Cleavage of the ribose and N-methylation yields 7-methylxanthosine. 7-Methylxanthosine in turn is the precursor to theobromine, which in turn is the precursor to ].<ref>{{cite book | vauthors = Ashihara H, Yokota T, Crozier A | title = New Light on Alkaloid Biosynthesis and Future Prospects | year = 2013 | chapter = Biosynthesis and catabolism of purine alkaloids | journal = Advances in Botanical Research | volume = 68 | pages = 111–138 | doi = 10.1016/B978-0-12-408061-4.00004-3 |isbn=9780124080614 }}</ref> | ||
| ==Pharmacology== | |||
| ==Therapeutic uses== | |||
| [[File:Caffeine metabolites.svg|thumb|250px|Caffeine is metabolized in the liver into three primary metabolites: | |||
| In modern ], theobromine is used as a ] (a blood vessel widener), a ] (urination aid), and ] ].<ref name="dictbiochem1943">{{cite book | title=Dictionary of Bio-Chemistry and Related Subjects | author=William Marias Malisoff | year=1943 | publisher=Philosophical Library | pages=311, 530, 573 | asin = B0006AQ0NU}}</ref> | |||
| ] (84%), theobromine (12%), and ] (4%){{Citation needed|date=August 2019}}|alt=A diagram featuring 4 skeletal chemical formulas. Top (caffeine) relates to similar compounds paraxanthine, theobromine and theophylline.]] | |||
| Even without dietary intake, theobromine may occur in the body as it is a product of the human metabolism of ], which is ] in the liver into 12% theobromine, 4% ], and 84% ].<ref name="pharmgkb">{{cite web | title = Caffeine | publisher = The Pharmacogenetics and Pharmacogenomics Knowledge Base | url = http://www.pharmgkb.org/do/serve?objId=PA448710&objCls=Drug#tabview=tab1 | access-date = 2011-01-08 | archive-date = 2010-11-24 | archive-url = https://web.archive.org/web/20101124155701/http://www.pharmgkb.org/do/serve?objId=PA448710&objCls=Drug#tabview=tab1 | url-status = dead }}</ref> | |||
| In the liver, theobromine is metabolized into ] and subsequently into ].<ref>{{cite journal | vauthors = Cornish HH, Christman AA | title = A study of the metabolism of theobromine, theophylline, and caffeine in man | journal = The Journal of Biological Chemistry | volume = 228 | issue = 1 | pages = 315–323 | date = September 1957 | doi = 10.1016/S0021-9258(18)70714-X | pmid = 13475320 | doi-access = free }}</ref> Important enzymes include ] and ].<ref>{{cite journal | vauthors = Gates S, Miners JO | title = Cytochrome P450 isoform selectivity in human hepatic theobromine metabolism | journal = British Journal of Clinical Pharmacology | volume = 47 | issue = 3 | pages = 299–305 | date = March 1999 | pmid = 10215755 | pmc = 2014222 | doi = 10.1046/j.1365-2125.1999.00890.x }}</ref> The elimination half life of theobromine is between 6 and 8 hours.<ref name="halflife1" /><ref name="halflife2" /> | |||
| Theobromine increases urine production. Because of this ] effect, and its ability to dilate blood vessels, theobromine has been used to treat high blood pressure.<ref name="chemistry.about.com">http://chemistry.about.com/od/factsstructures/a/theobromine-chemistry.htm</ref> ''The American Journal of Clinical Nutrition'' notes that historic use of theobromine as a treatment for other circulatory problems including ], certain ]s, ], and ] should be considered in future studies.<ref name="AJCN">{{cite journal | title=Effects of theobromine should be considered in future studies | last=Kelly | first=Caleb J |date=August 2005 | journal=American Journal of Clinical Nutrition |volume=82 |issue=2 | pages=486–7; author reply 487–8 | pmid=16087999}}</ref> | |||
| Unlike caffeine, which is highly water-soluble, theobromine is only slightly water-soluble and is more fat soluble, and thus peaks more slowly in the blood. While caffeine peaks after only 30 minutes, theobromine requires 2–3 hours to peak.<ref>{{cite journal | vauthors = Mumford GK, Benowitz NL, Evans SM, Kaminski BJ, Preston KL, Sannerud CA, Silverman K, Griffiths RR | title = Absorption rate of methylxanthines following capsules, cola and chocolate | journal = European Journal of Clinical Pharmacology | volume = 51 | issue = 3–4 | pages = 319–325 | date = 1 December 1996 | pmid = 9010706 | doi = 10.1007/s002280050205 | s2cid = 8405909 }}</ref> | |||
| Following its discovery in the late 19th century, theobromine was put to use by 1916, when it was recommended by the publication ''Principles of Medical Treatment'' as a treatment for ] (excessive liquid in parts of the body), ] ] attacks, and degenerative angina.<ref name="princimedtreat-1916">{{cite book | title=Principles of medical treatment | author=George Cheever Shattuck | pages=15, 39, 41 | year=1916 | publisher=W.M. Leonard }}</ref> | |||
| The primary mechanism of action for theobromine inside the body is inhibition of ] receptors.<ref name=Hbk/> Its effect as a ]<ref name="PDEs-Essayan">{{cite journal | vauthors = Essayan DM | title = Cyclic nucleotide phosphodiesterases | journal = The Journal of Allergy and Clinical Immunology | volume = 108 | issue = 5 | pages = 671–680 | date = November 2001 | pmid = 11692087 | doi = 10.1067/mai.2001.119555 | doi-access = free }}</ref> is thought to be small.<ref name=Hbk/> | |||
| In the human body, theobromine levels are halved between 6–10 hours after consumption.<ref name="chemistry.about.com"/> | |||
| Theobromine has also been used in ] experiments involving ] and ]s. A decreased ] weight was noted in rabbits following ], but not after other administration of theobromine. Birth defects were not seen in rats.<ref>{{cite journal | author=Rambali B, Andel I van, Schenk E, Wolterink G, Werken G van de, Stevenson H, Vleeming W | title= | journal=RIVM | year=2002 | url=http://rivm.nl/bibliotheek/rapporten/650270002.pdf | format=PDF |issue=report 650270002/2002}}- The National Institute for Public Health and the Environment (Netherlands)</ref> Possible future uses of theobromine in such fields as ] prevention have been patented.<ref>{{Ref patent | number=6693104 | title=Theobromine with an anti-carcinogenic activity | gdate=2004-02-17 | country=US }}</ref> | |||
| ==Pharmacology== | |||
| Even without dietary intake, theobromine may occur in the body as it is a product of the human metabolism of ], which is ] in the liver into 12% theobromine, 4% ], and 84% ].<ref name="pharmgkb">{{cite web | title = Caffeine | publisher = The Pharmacogenetics and Pharmacogenomics Knowledge Base | url = http://www.pharmgkb.org/do/serve?objId=PA448710&objCls=Drug#tabview=tab1 | accessdate = 2011-01-08 }}</ref> | |||
| In the liver, theobromine is metabolized into ] and subsequently into ].<ref>{{Cite journal | title=A Study of the Metabolism of Theobromine, Theophylline, and Caffeine in Man | author=Herbert H. Cornish and A. A. Christman | year=1957 | publisher=Department of Biological Chemistry, Medical School, University of Michigan | location=Ann Arbor, Michigan }}</ref> Important enzymes include ] and ].<ref>{{cite journal| journal=Br J Clin Pharmacol|date=March 1999|volume=47|issue=3| pages=299–305| author=Gates S, Miners JO| title=Cytochrome P450 isoform selectivity in human hepatic theobromine metabolism| pmid=10215755 | doi = 10.1046/j.1365-2125.1999.00890.x| pmc=2014222}}</ref> | |||
| Like other methylated ], theobromine is both a: | |||
| # competitive nonselective ],<ref name="PDEs-Essayan">{{cite journal | author=Essayan DM. | title=Cyclic nucleotide phosphodiesterases | journal=J Allergy Clin Immunol. | year=2001 | pages=671–80 |volume=108 |issue=5 | pmid=11692087 | doi=10.1067/mai.2001.119555 }}</ref> which raises intracellular ], activates ], ]<ref name="PTX-Deree">{{cite journal | author=Deree J, Martins JO, Melbostad H, Loomis WH, Coimbra R. | title=Insights into the regulation of TNF-alpha production in human mononuclear cells: the effects of non-specific phosphodiesterase inhibition | journal=Clinics (São Paulo). | year=2008 | pages=321–8 |volume=63 |issue=3 | pmid=18568240 | doi=10.1590/S1807-59322008000300006 | pmc=2664230 }}</ref><ref name="pmid9927365">{{cite journal |author=Marques LJ, Zheng L, Poulakis N, Guzman J, Costabel U |title=Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages |journal=Am. J. Respir. Crit. Care Med. |volume=159 |issue=2 |pages=508–11 |date=February 1999 |pmid=9927365 |doi= 10.1164/ajrccm.159.2.9804085|url=http://ajrccm.atsjournals.org/cgi/pmidlookup?view=long&pmid=9927365}}</ref> and ]<ref name="LT-Peters-Golden">{{cite journal | author=Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. | title=Leukotrienes: underappreciated mediators of innate immune responses | journal=J Immunol. | year=2005 | pages=589–94 |volume=174 |issue=2 | pmid=15634873 | url=http://www.jimmunol.org/cgi/content/full/174/2/589 | doi=10.4049/jimmunol.174.2.589}}</ref> synthesis, and ] and ]<ref name="LT-Peters-Golden"/> and | |||
| # nonselective ] antagonist.<ref name="AR-Daly">{{cite journal | author=Daly JW, Jacobson KA, Ukena D. | title=Adenosine receptors: development of selective agonists and antagonists | journal=Prog Clin Biol Res. | year=1987 | pages=41–63 |volume=230 |issue=1 | pmid=3588607 }}</ref> | |||
| As a ], theobromine prevents the ] enzymes from converting the active cAMP to an inactive form.<ref name="omd-pdaseinhibitor">* {{cite web | url=http://cancerweb.ncl.ac.uk/cgi-bin/omd?phosphodiesterase | title=Phosphodiesterase | publisher=On-Line Medical Dictionary | accessdate=2007-02-23 }}<br/> {{cite web | url=http://cancerweb.ncl.ac.uk/cgi-bin/omd?inhibitors | title=Inhibitor | publisher=On-Line Medical Dictionary | accessdate=2007-02-23 }}</ref> cAMP works as a ] in many ]- and ]-controlled metabolic systems, such as the breakdown of ]. When the inactivation of cAMP is inhibited by a compound such as theobromine, the effects of the neurotransmitter or hormone that stimulated the production of cAMP are much longer-lived. In general, the net result is a stimulatory effect.<ref name="lehningerbiochem">{{cite book | title=Lehninger Principles of Biochemistry | author=David L. Nelson, Michael M. Cox | year=2005 | publisher=W.H. Freeman and Company | pages=435–439 | isbn = 0-7167-4339-6 }}</ref> | |||
| ==Effects== | ==Effects== | ||
| {{see also|Theobromine poisoning}} | |||
| ===Humans=== | ===Humans=== | ||
| Theobromine is a heart stimulator and diuretic but has no significant stimulant effect on the human central nervous system.<ref name=pubchem/> It is a ] and causes relaxation of ].<ref name=pubchem/> It is available as a ] in South Korea.<ref>{{cite web|url=https://www.health.kr/searchDrug/result_drug.asp?drug_cd=A11AKP08G4675|title=Anycough Cap 300mg|publisher=Korea Pharmaceutical Information Center|access-date=8 January 2025|archive-date=8 August 2024|archive-url=https://web.archive.org/web/20240808134400/https://www.health.kr/searchDrug/result_drug.asp?drug_cd=A11AKP08G4675|url-status=live}}</ref> The amount of theobromine found in chocolate is small enough that chocolate can, in general, be safely consumed by humans. | |||
| ] | |||
| The amount of theobromine found in chocolate is small enough that chocolate can, in general, be safely consumed by humans. ] may result from the chronic or acute consumption of large quantities, especially in the elderly.<ref> in the ]</ref> | |||
| Compared with caffeine, theobromine is weaker in both its inhibition of ] ] and its ] of ].<ref name=pubchem/><ref name="therapeutics">{{cite book | veditors = Hardman J, Limbird L | title=Goodman & Gilman's the pharmacological basis of therapeutics, 10th ed. | publisher=McGraw-Hill | location=New York | year=2001 | page=745 | isbn=978-0-07-135469-1}}</ref> The potential ]y effect of theobromine is seen only at amounts much higher than what people normally would consume in a typical diet including chocolate.<ref>{{cite web|url=https://www.drugbank.ca/drugs/DB01412|title=Theobromine|publisher=DrugBank.ca|access-date=3 November 2018|archive-date=13 November 2018|archive-url=https://web.archive.org/web/20181113075732/https://www.drugbank.ca/drugs/DB01412|url-status=dead}}</ref> | |||
| ====Toxicity==== | |||
| As it is a myocardial stimulant as well as a vasodilator, it increases heartbeat, and also dilates blood vessels, causing a reduced ].<ref name="arecapatent">{{Ref patent | country=US | number=20050089584 | title=Methods and compositions for oral delivery of Areca and mate' or theobromine | gdate=2005-04-28 }}</ref> A 2005 paper published suggested that the decrease in blood pressure may be caused by ].<ref name="AJCN"/> Its draining effect allows it to be used to treat ], which leads to and is exacerbated by an excessive accumulation of fluid in the body.<ref name="arecapatent"/> | |||
| At doses of 0.8–1.5 g/day (50–100 g cocoa), sweating, trembling and severe headaches were noted, with limited mood effects found at 250 mg/day.<ref>{{cite web|url=https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb%3A%40term+%40DOCNO+7332#permalink|title=3,7-Dimethylxanthine (Theobromine)|publisher=Toxnet, US National Library of Medicine|date=1 December 2017|access-date=13 November 2018|archive-date=7 October 2018|archive-url=https://web.archive.org/web/20181007145619/https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@DOCNO+7332#permalink|url-status=live}}</ref> | |||
| Also, chocolate may be a factor for ] in some people because theobromine may affect the ] muscle in a way that permits stomach acids to enter the ].<ref name="Latif">{{cite journal | vauthors = Latif R | title = Chocolate/cocoa and human health: a review | journal = The Netherlands Journal of Medicine | volume = 71 | issue = 2 | pages = 63–68 | date = March 2013 | pmid = 23462053 | url = http://www.njmonline.nl/getpdf.php?id=1269 }}</ref> | |||
| A 2004 study published by ] concluded that theobromine has an ] (cough-reducing) effect superior to ] by suppressing ] activity.<ref name=PMID_15548587>{{cite journal | author = Usmani, Omar S.; Belvisi, Maria G.; Patel, Hema J.; Crispino, Natascia; Birrell Mark A.; Korbonits, Márta; Korbonits, Dezső; Barnes, Peter J. | title = Theobromine inhibits sensory nerve activation and cough | journal = ] Journal | volume = 19 | issue = 2 | pages = 231–3 | pmid = 15548587 | url = http://www.fasebj.org/cgi/reprint/04-1990fjev1 | doi = 10.1096/fj.04-1990fje | accessdate = 2008-07-04 | date = November 17, 2004 | quote = The present study demonstrates that theobromine, a methylxanthine derivative present in cocoa, effectively inhibits ]-induced cough in ] ]. Furthermore, in a randomized, double-blind, placebo controlled study in man, theobromine suppresses capsaicin-induced cough with no adverse effects. We also demonstrate that theobromine directly inhibits ]-induced sensory nerve depolarization of guinea-pig and human vagus nerve suggestive of an inhibitory effect on ] activation.}}</ref> In the study, 1000 mg theobromine (equivalent to ~71g dark chocolate) significantly increased the threshold of ] concentration required to induce coughs when compared with a placebo.<ref name=PMID_15548587/> A drug, called BC1036, is being developed by the private UK company infirst Healthcare and it uses theobromine to treat ].<ref>{{cite news |title='Chocolate cough remedy' in sight |publisher=BBC News |date=2010-12-21 |url=http://www.bbc.co.uk/news/health-12048275 |accessdate=2010-12-21}}</ref> Theobromine is helpful in treating ], since it relaxes the ]s, including the ones found in the ].<ref name="asthma">{{cite book | author=Irwin J. Polk | title=All about Asthma: Stop Suffering and Start Living | year=1997 | location=New York | publisher=Insight Books | page=100 | isbn = 0-306-45569-2 }}</ref> | |||
| A study conducted in Utah between 1983 and 1986, and published in 1993, showed a possible association between theobromine and an increased risk of ] in older men.<ref name=PMID_8280834>{{cite journal | author = Slattery, Martha L.; West, Dee W. | title = Smoking, alcohol, coffee, tea, caffeine, and theobromine: risk of prostate cancer in Utah (United States) | journal = Cancer Causes Control | volume = 4 | issue = 6 | pages = 559–63 | year = 1993 | pmid = 8280834 | quote = Compared with men with very low levels of theobromine intake, older men consuming 11 to 20 and over 20 mg of theobromine per day were at increased risk of prostate cancer (odds ratio- for all tumors = 2.06, 95 percent confidence interval = 1.33-3.20, and OR = 1.47, CI = 0.99-2.19, respectively; OR for aggressive tumors -- 1.90, CI = 0.90-3.97, and OR -- 1.74, CI -- 0.91-3.32, respectively) | doi = 10.1007/BF00052432}}</ref> This association was not found to be linear for aggressive tumors.<ref name=PMID_8280834/> The association may be spurious, but is plausible.<ref name=PMID_8280834/> Prenatal and infant exposure to theobromine appeared possibly associated with ] and testicular cancer in one population study.<ref>{{cite journal | pmid = 19440400 | doi=10.3390/ijerph6020578 | volume=6 | issue=2 | title=Correlation analysis of cocoa consumption data with worldwide incidence rates of testicular cancer and hypospadias | pmc=2672359 |date=February 2009 | author=Giannandrea F | journal=Int J Environ Res Public Health | pages=568–78}}</ref> | |||
| As with caffeine, theobromine can cause sleeplessness, tremors, restlessness, anxiety, as well as contribute to ].<ref name="asthma"/> Additional side effects include ], ], ], and withdrawal headaches. | |||
| ===Animals=== | ===Animals=== | ||
| Theobromine is the reason chocolate is poisonous to dogs. Dogs and other animals that ] theobromine (found in chocolate) more slowly<ref>{{cite web|url=http://www.merckmanuals.com/vet/toxicology/food_hazards/chocolate.html|title=Chocolate – Toxicology – Merck Veterinary Manual|access-date=23 December 2017|archive-date=12 July 2014|archive-url=https://web.archive.org/web/20140712005049/http://www.merckmanuals.com/vet/toxicology/food_hazards/chocolate.html|url-status=live}}</ref> can succumb to theobromine poisoning from as little as {{cvt|50|g|oz}} of ] for a smaller dog and {{cvt|400|g|oz}}, or around nine {{convert|1.55|oz|order=flip|adj=on}} small milk chocolate bars, for an average-sized dog. The concentration of theobromine in dark chocolates (about {{cvt|10|g/kg}}) is up to 10 times that of milk chocolate ({{cvt|1|to|5|g/kg}}), meaning dark chocolate is far more toxic to dogs per unit weight or volume than milk chocolate.{{Cn|date=May 2024}} | |||
| The ] of theobromine for dogs is {{cvt|100|–|200|mg/kg|}}; therefore, a {{cvt|10|kg|}} dog would need to consume a minimum of {{cvt|200|g|}} of the most theobromine-rich ({{cvt|5|g/kg|}}) dark chocolate, or a maximum of {{cvt|1|kg|}} (of theobromine-rich milk chocolate), to have a 50% chance of receiving a lethal dose. However, even {{cvt|40|g|}} of milk chocolate may induce vomiting and diarrhea.<ref>{{cite web |last1=Gwaltney-Brant |first1=Sharon |title=Chocolate Toxicosis in Animals |url=https://www.merckvetmanual.com/toxicology/food-hazards/chocolate-toxicosis-in-animals |website=Merck Veterinary Manual |publisher=Merck & Co., Inc. |access-date=24 December 2023}}</ref> | |||
| Animals that ] theobromine (found in chocolate) more slowly, such as dogs , can succumb to ] from as little as {{convert|50|g|oz}} of milk chocolate for a smaller dog and {{convert|400|g|oz}}, or around nine 1.55 oz small milk chocolate ], for an average-sized dog. It should be observed the concentration of theobromine in dark chocolates (approximately 10 g/kg) is up to 10 times that of milk chocolate (1-5 g/kg) - meaning dark chocolate is far more toxic to dogs per unit weight or volume than milk chocolate. | |||
| The same risk is reported for cats as well , although cats are less likely to ingest sweet food, with most cats having no ]. Complications include digestive issues, dehydration, excitability, and a slow heart rate. Later stages of theobromine poisoning include ]-like ]s and death. If caught early on, theobromine poisoning is treatable.<ref name="healthwatchcanines">{{Cite news | title=HEALTH WATCH: How to Avoid a Canine Chocolate Catastrophe! | newspaper=The News Letter | location=Belfast, Northern Ireland | date=2005-03-01}}</ref> Although not usual, the effects of theobromine poisoning, as stated, can become fatal. | |||
| The toxicity for birds is not known, but it is typically assumed that it is toxic to birds.<ref>B. Harvey, .</ref> | |||
| The same risk is reported for cats as well,<ref>{{cite web |url=http://aspcapro.org/sites/pro/files/m-toxbrief_0201_0.pdf |title=Chocolate intoxication |vauthors=Gwaltney-Brant S |publisher=] |website=aspcapro.org |access-date=23 December 2017 |archive-date=8 February 2017 |archive-url=https://web.archive.org/web/20170208145634/http://aspcapro.org/sites/pro/files/m-toxbrief_0201_0.pdf |url-status=dead}}</ref> although cats are less likely to ingest sweet food, as cats lack ].<ref name=wired>{{cite magazine |url=https://wired.com/2013/02/the-poisonous-nature-of-chocolate |title=The Poisonous Chemistry of Chocolate |date=14 February 2013 |magazine=] |access-date=12 March 2017 |archive-date=8 February 2017 |archive-url=https://web.archive.org/web/20170208145832/https://www.wired.com/2013/02/the-poisonous-nature-of-chocolate/ |url-status=live}}</ref> Complications include digestive issues, dehydration, excitability, and a slow heart rate. Later stages of theobromine poisoning include ]-like ]s and death. If caught early on, theobromine poisoning is treatable.<ref name="healthwatchcanines">{{cite news | title=HEALTH WATCH: How to Avoid a Canine Chocolate Catastrophe! | newspaper=The News Letter | location=Belfast, Northern Ireland | date=2005-03-01}}</ref> Although not common, the effects of theobromine poisoning can be fatal.<ref name="wired" /> | |||
| ===Gene mutation in mammalian cells=== | |||
| Theobromine is known to induce ] ]s in lower ]s and ]. In 1991 and 1997, research by the ] had shown that genetic mutations occurred in higher eukaryotic cells, specifically cultured ] cells. But despite this, theobromine was still listed as safe for human consumption due to inadequate evidence of carcinogenicity.<ref>{{cite web | author=International Agency for Research on Cancer | title=Volume 51: Coffee, Tea, Mate, Methylxanthines and Methylglyoxal - Theobromine | url=http://monographs.iarc.fr/ENG/Monographs/vol51/volume51.pdf | format=PDF | work=IARC Monographs on the Evaluation of Carcinogenic Risks to Humans | publisher=] | date=November 17, 1991 | accessdate=2006-09-19 | authorlink=International Agency for Research on Cancer}}</ref> | |||
| ==See also== | == See also == | ||
| * ] | * ] | ||
| * ] | |||
| ==References== | == References == | ||
| {{Reflist |
{{Reflist}} | ||
| ==Further reading== | |||
| {{Refbegin}} | |||
| * {{cite book | last = Bender | first = David A. | author2 = Arnold E. Bender | title=A Dictionary of Food and Nutrition | year=1995 | publisher=Oxford University Press | location=Oxford | isbn = 0-19-860961-2 }} | |||
| {{Refend}} | |||
| :* from the ] | |||
| {{Commons category|Theobromine}} | |||
| {{Chocolate}} | |||
| {{Diuretics}} | {{Diuretics}} | ||
| {{Asthma and copd rx}} | {{Asthma and copd rx}} | ||
| {{Adenosinergics}} | {{Adenosinergics}} | ||
| {{Phosphodiesterase inhibitors}} | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
Latest revision as of 11:25, 8 January 2025
Bitter alkaloid of the cacao plantNot to be confused with bromine. Pharmaceutical compound
 | |
 | |
| Clinical data | |
|---|---|
| Other names | xantheose diurobromine 3,7-dimethylxanthine 3,7-dihydro-3,7-dimethyl-1H-purine-2,6-dione |
| Dependence liability | None |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic demethylation and oxidation |
| Elimination half-life | 6–8 hours |
| Excretion | Renal (10% unchanged, rest as metabolites) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.359 |
| Chemical and physical data | |
| Formula | C7H8N4O2 |
| Molar mass | 180.167 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
| Identifiers | |
|---|---|
| ECHA InfoCard | 100.001.359 |
| CompTox Dashboard (EPA) | |
| Properties | |
| Appearance | white solid |
| Density | 1.524 g/cm |
| Melting point | 351 °C (664 °F; 624 K) |
| Solubility in water | 330 mg/L) |
Theobromine, also known as xantheose, is the principal alkaloid of Theobroma cacao (cacao plant). Theobromine is slightly water-soluble (330 mg/L) with a bitter taste. In industry, theobromine is used as an additive and precursor to some cosmetics. It is found in chocolate, as well as in a number of other foods, including tea (Camellia sinensis), some American hollies (yaupon and guayusa) and the kola nut. It is a white or colourless solid, but commercial samples can appear yellowish.
Structure
Theobromine is a flat molecule, a derivative of purine and an isomer of theophylline. It is also classified as a dimethyl xanthine. Related compounds include theophylline, caffeine, paraxanthine, and 7-methylxanthine, each of which differ in the number or placement of the methyl groups.
History
Theobromine was first discovered in 1841 in cacao beans by the chemist A. Woskresensky. Synthesis of theobromine from xanthine was first reported in 1882 by Hermann Emil Fischer.
Etymology
Theobromine is derived from Theobroma, the name of the genus of the cacao tree, with the suffix -ine given to alkaloids and other basic nitrogen-containing compounds. That name in turn is made up of the Greek roots theo ("god") and broma ("food"), meaning "food of the gods".
Despite its name, the compound contains no bromine, which is based on Greek bromos ("stench").
Sources

Theobromine is the primary alkaloid found in cocoa and chocolate. Cocoa butter only contains trace amounts of theobromine. There are usually higher concentrations in dark than in milk chocolate.
There are approximately 60 milligrams (1 grain) of theobromine in 28 grams (1 oz) of milk chocolate, while the same amount of dark chocolate contains about 200 milligrams (3 grains). Cocoa beans naturally contain approximately 1% theobromine.
Plant species and components with substantial amounts of theobromine are:
- Theobroma cacao – seed and seed coat
- Theobroma bicolor – seed coat
- Ilex paraguariensis – leaf
- Ilex guayusa – leaf
- Ilex vomitoria – leaf
- Camellia sinensis – leaf
Theobromine can also be found in trace amounts in the kola nut, the guarana berry, yerba mate (Ilex paraguariensis), and the tea plant.
The mean theobromine concentrations in cocoa and carob products are:
| Item | Mean theobromine per 100 g |
|---|---|
| Cocoa powder | 2060 mg |
| Cocoa beverages | 266 mg |
| Chocolate toppings | 195 mg |
| Chocolate bakery products | 147 mg |
| Cocoa cereals | 69.5 mg |
| Chocolate ice creams | 62.1 mg |
| Chocolate milks | 22.6 mg |
| Carob products | 0.00–50.4 mg |
Biosynthesis
Theobromine is a purine alkaloid derived from xanthosine, a nucleoside. Cleavage of the ribose and N-methylation yields 7-methylxanthosine. 7-Methylxanthosine in turn is the precursor to theobromine, which in turn is the precursor to caffeine.
Pharmacology

Even without dietary intake, theobromine may occur in the body as it is a product of the human metabolism of caffeine, which is metabolised in the liver into 12% theobromine, 4% theophylline, and 84% paraxanthine.
In the liver, theobromine is metabolized into xanthine and subsequently into methyluric acid. Important enzymes include CYP1A2 and CYP2E1. The elimination half life of theobromine is between 6 and 8 hours.
Unlike caffeine, which is highly water-soluble, theobromine is only slightly water-soluble and is more fat soluble, and thus peaks more slowly in the blood. While caffeine peaks after only 30 minutes, theobromine requires 2–3 hours to peak.
The primary mechanism of action for theobromine inside the body is inhibition of adenosine receptors. Its effect as a phosphodiesterase inhibitor is thought to be small.
Effects
See also: Theobromine poisoningHumans
Theobromine is a heart stimulator and diuretic but has no significant stimulant effect on the human central nervous system. It is a bronchodilator and causes relaxation of vascular smooth muscle. It is available as a prescription drug in South Korea. The amount of theobromine found in chocolate is small enough that chocolate can, in general, be safely consumed by humans.
Compared with caffeine, theobromine is weaker in both its inhibition of cyclic nucleotide phosphodiesterases and its antagonism of adenosine receptors. The potential phosphodiesterase inhibitory effect of theobromine is seen only at amounts much higher than what people normally would consume in a typical diet including chocolate.
Toxicity
At doses of 0.8–1.5 g/day (50–100 g cocoa), sweating, trembling and severe headaches were noted, with limited mood effects found at 250 mg/day.
Also, chocolate may be a factor for heartburn in some people because theobromine may affect the esophageal sphincter muscle in a way that permits stomach acids to enter the esophagus.
Animals
Theobromine is the reason chocolate is poisonous to dogs. Dogs and other animals that metabolize theobromine (found in chocolate) more slowly can succumb to theobromine poisoning from as little as 50 g (1.8 oz) of milk chocolate for a smaller dog and 400 g (14 oz), or around nine 44-gram (1.55 oz) small milk chocolate bars, for an average-sized dog. The concentration of theobromine in dark chocolates (about 10 g/kg (0.16 oz/lb)) is up to 10 times that of milk chocolate (1 to 5 g/kg (0.016 to 0.080 oz/lb)), meaning dark chocolate is far more toxic to dogs per unit weight or volume than milk chocolate.
The median lethal dose of theobromine for dogs is 100–200 mg/kg (0.0016–0.0032 oz/lb); therefore, a 10 kg (22 lb) dog would need to consume a minimum of 200 g (7.1 oz) of the most theobromine-rich (5 g/kg (0.080 oz/lb)) dark chocolate, or a maximum of 1 kg (2.2 lb) (of theobromine-rich milk chocolate), to have a 50% chance of receiving a lethal dose. However, even 40 g (1.4 oz) of milk chocolate may induce vomiting and diarrhea.
The same risk is reported for cats as well, although cats are less likely to ingest sweet food, as cats lack sweet taste receptors. Complications include digestive issues, dehydration, excitability, and a slow heart rate. Later stages of theobromine poisoning include epileptic-like seizures and death. If caught early on, theobromine poisoning is treatable. Although not common, the effects of theobromine poisoning can be fatal.
See also
References
- ^ Drouillard DD, Vesell ES, Dvorchik BH (March 1978). "Studies on theobromine disposition in normal subjects. Alterations induced by dietary abstention from or exposure to methylxanthines". Clinical Pharmacology and Therapeutics. 23 (3): 296–302. doi:10.1002/cpt1978233296. PMID 627135. S2CID 10519385.
- ^ Lelo A, Birkett DJ, Robson RA, Miners JO (August 1986). "Comparative pharmacokinetics of caffeine and its primary demethylated metabolites paraxanthine, theobromine and theophylline in man". British Journal of Clinical Pharmacology. 22 (2): 177–182. doi:10.1111/j.1365-2125.1986.tb05246.x. PMC 1401099. PMID 3756065.
- ^ Ford KA, Ebisuzaki Y, Boyle PD (1998). "Methylxanthines. II. Anhydrous Theobromine". Acta Crystallographica Section C Crystal Structure Communications. 54 (12): 1980–1983. Bibcode:1998AcCrC..54.1980F. doi:10.1107/S0108270198009469.
- ^ "Theobromine". PubChem, US National Library of Medicine. 27 August 2022. Retrieved 3 September 2022.
- ^ Smit HJ (2011). "Theobromine and the Pharmacology of Cocoa". Methylxanthines. Handbook of Experimental Pharmacology. Vol. 200. pp. 201–234. doi:10.1007/978-3-642-13443-2_7. ISBN 978-3-642-13442-5. PMID 20859797.
- "Theophylline". PubChem, US National Library of Medicine. 26 August 2023. Retrieved 2 September 2023.
- Baer DM, Pinkston EM (1997). Environment and Behavior. Westview Press. p. 200. ISBN 978-0813331591.
- von Bibra E, Ott J (1995). Plant Intoxicants: A Classic Text on the Use of Mind-Altering Plants. Inner Traditions / Bear & Co. pp. 67–. ISBN 978-0-89281-498-5. Archived from the original on 2019-09-18. Retrieved 2015-12-12.
- Woskresensky A (1842). "Über das Theobromin". Liebigs Annalen der Chemie und Pharmacie. 41: 125–127. doi:10.1002/jlac.18420410117. Archived from the original on 2016-06-10. Retrieved 2015-12-12.
- Thorpe TE (1902). Essays in Historical Chemistry. The MacMillan Company.
- Fischer, Emil (1882). "Umwandlung des Xanthin in Theobromin und Caffein". Berichte der Deutschen Chemischen Gesellschaft. 15 (1): 453–456. doi:10.1002/cber.18820150194. Archived (PDF) from the original on 2019-05-05. Retrieved 2019-09-09.
- Fischer E (1882). "Über Caffein, Theobromin, Xanthin und Guanin". Justus Liebigs Annalen der Chemie. 215 (3): 253–320. doi:10.1002/jlac.18822150302.
- "-ine". The American Heritage Dictionary of the English Language, Fourth Edition. Houghton Mifflin Company. 2004. ISBN 978-0-395-71146-0. Archived from the original on 2016-03-03. Retrieved 2007-02-23.
- Bennett AW, Bealer BK (2002). The World of Caffeine: The Science and Culture of the World's Most Popular Drug. Routledge, New York. ISBN 978-0-415-92723-9. (note: the book incorrectly states that the name "theobroma" is derived from Latin)
- "AmerMed cocoa extract with 10% theobromine". AmerMed. Archived from the original on 2015-08-11. Retrieved 2008-04-13.
- "USDA Nutrient database, entries for milk chocolate". Archived from the original on 2017-07-08. Retrieved 2012-12-29.
- "USDA Nutrient database, entries for dark chocolate". Retrieved 2012-11-07.
- Kuribara H, Tadokoro S (April 1992). "Behavioral effects of cocoa and its main active compound theobromine: evaluation by ambulatory activity and discrete avoidance in mice". Arukoru Kenkyu to Yakubutsu Izon = Japanese Journal of Alcohol Studies & Drug Dependence. 27 (2): 168–179. PMID 1586288.
- "Theobromine content in plant sources". Dr. Duke's Phytochemical and Ethnobotanical Databases, United States Department of Agriculture. 6 February 2019. Archived from the original on 8 May 2019. Retrieved 9 March 2019.
- Crown PL, Emerson TE, Gu J, Hurst WJ, Pauketat TR, Ward T (August 2012). "Ritual Black Drink consumption at Cahokia". Proc. Natl. Acad. Sci. U.S.A. 109 (35): 13944–9. doi:10.1073/pnas.1208404109. PMC 3435207. PMID 22869743.
- Schuster J, Mitchell ES (March 2019). "More than just caffeine: psychopharmacology of methylxanthine interactions with plant-derived phytochemicals". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 89: 263–274. doi:10.1016/j.pnpbp.2018.09.005. PMID 30213684. S2CID 52274913.
- Prance G, Nesbitt M (2004). The Cultural History of Plants. New York: Routledge. pp. 137, 175, 178–180. ISBN 978-0-415-92746-8.
- "FoodData Central". fdc.nal.usda.gov.
- Craig WJ, Nguyen TT (1984). "Caffeine and theobromine levels in cocoa and carob products". Journal of Food Science. 49 (1): 302–303. doi:10.1111/j.1365-2621.1984.tb13737.x.
Mean theobromine and caffeine levels respectively, were 0.695 mg/g and 0.071 mg/g in cocoa cereals; 1.47 mg/g and 0.152 mg/g in chocolate bakery products; 1.95 mg/g and 0.138 mg/g in chocolate toppings; 2.66 mg/g and 0.208 mg/g in cocoa beverages; 0.621 mg/g and 0.032 mg/g in chocolate ice creams; 0.226 mg/g and 0.011 mg/g in chocolate milks; 74.8 mg/serving and 6.5 mg/serving in chocolate puddings.... Theobromine and caffeine levels in carob products ranged from 0–0.504 mg/g and 0-0.067 mg/g, respectively.
- Ashihara H, Yokota T, Crozier A (2013). "Biosynthesis and catabolism of purine alkaloids". New Light on Alkaloid Biosynthesis and Future Prospects. Vol. 68. pp. 111–138. doi:10.1016/B978-0-12-408061-4.00004-3. ISBN 9780124080614.
{{cite book}}:|journal=ignored (help) - "Caffeine". The Pharmacogenetics and Pharmacogenomics Knowledge Base. Archived from the original on 2010-11-24. Retrieved 2011-01-08.
- Cornish HH, Christman AA (September 1957). "A study of the metabolism of theobromine, theophylline, and caffeine in man". The Journal of Biological Chemistry. 228 (1): 315–323. doi:10.1016/S0021-9258(18)70714-X. PMID 13475320.
- Gates S, Miners JO (March 1999). "Cytochrome P450 isoform selectivity in human hepatic theobromine metabolism". British Journal of Clinical Pharmacology. 47 (3): 299–305. doi:10.1046/j.1365-2125.1999.00890.x. PMC 2014222. PMID 10215755.
- Mumford GK, Benowitz NL, Evans SM, Kaminski BJ, Preston KL, Sannerud CA, et al. (1 December 1996). "Absorption rate of methylxanthines following capsules, cola and chocolate". European Journal of Clinical Pharmacology. 51 (3–4): 319–325. doi:10.1007/s002280050205. PMID 9010706. S2CID 8405909.
- Essayan DM (November 2001). "Cyclic nucleotide phosphodiesterases". The Journal of Allergy and Clinical Immunology. 108 (5): 671–680. doi:10.1067/mai.2001.119555. PMID 11692087.
- "Anycough Cap 300mg". Korea Pharmaceutical Information Center. Archived from the original on 8 August 2024. Retrieved 8 January 2025.
- Hardman J, Limbird L, eds. (2001). Goodman & Gilman's the pharmacological basis of therapeutics, 10th ed. New York: McGraw-Hill. p. 745. ISBN 978-0-07-135469-1.
- "Theobromine". DrugBank.ca. Archived from the original on 13 November 2018. Retrieved 3 November 2018.
- "3,7-Dimethylxanthine (Theobromine)". Toxnet, US National Library of Medicine. 1 December 2017. Archived from the original on 7 October 2018. Retrieved 13 November 2018.
- Latif R (March 2013). "Chocolate/cocoa and human health: a review". The Netherlands Journal of Medicine. 71 (2): 63–68. PMID 23462053.
- "Chocolate – Toxicology – Merck Veterinary Manual". Archived from the original on 12 July 2014. Retrieved 23 December 2017.
- Gwaltney-Brant S. "Chocolate Toxicosis in Animals". Merck Veterinary Manual. Merck & Co., Inc. Retrieved 24 December 2023.
- Gwaltney-Brant S. "Chocolate intoxication" (PDF). aspcapro.org. ASPCA. Archived from the original (PDF) on 8 February 2017. Retrieved 23 December 2017.
- ^ "The Poisonous Chemistry of Chocolate". Wired. 14 February 2013. Archived from the original on 8 February 2017. Retrieved 12 March 2017.
- "HEALTH WATCH: How to Avoid a Canine Chocolate Catastrophe!". The News Letter. Belfast, Northern Ireland. 2005-03-01.
| Diuretics (C03) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sulfonamides (and etacrynic acid) |
| ||||||||
| Potassium-sparing (at CD) |
| ||||||||
| Osmotic diuretics (PT, DL) | |||||||||
| Vasopressin receptor inhibitors (DCT and CD) | |||||||||
| Other | |||||||||
| Combination products | |||||||||
| |||||||||
| Purine receptor modulators | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor (ligands) |
| ||||||||||
| Transporter (blockers) |
| ||||||||||
| Enzyme (inhibitors) |
| ||||||||||
| Others |
| ||||||||||
| See also: Receptor/signaling modulators | |||||||||||
| Phosphodiesterase inhibitors | |
|---|---|
| PDE1 | |
| PDE2 | |
| PDE3 | |
| PDE4 |
|
| PDE5 | |
| PDE7 | |
| PDE9 | |
| PDE10 | |
| PDE11 | BC11-38 |
| Non-selective | |
| Unsorted | |
| See also: Receptor/signaling modulators | |