This is an old revision of this page, as edited by Luckas-bot (talk | contribs) at 23:49, 4 November 2011 (r2.7.1) (Robot: Adding vi:Phốtphin). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 23:49, 4 November 2011 by Luckas-bot (talk | contribs) (r2.7.1) (Robot: Adding vi:Phốtphin)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) This article is about the chemical. For the visual phenomenon, see phosphene. "Phosphane" redirects here. For pentavalent organophosphorus compounds, see phosphorane. Not to be confused with phosgene.
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Phosphane | |||
| Other names
Phosphamine Phosphorus trihydride Phosphorated hydrogen | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.328 | ||
| EC Number |
| ||
| PubChem CID | |||
| RTECS number |
| ||
| UN number | 2199 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | PH3 | ||
| Molar mass | 33.99758 g/mol | ||
| Appearance | colorless gas | ||
| Density | 1.379 g/l, gas (25 °C) | ||
| Melting point | −132.8 °C (−207.0 °F; 140.3 K) | ||
| Boiling point | −87.7 °C (−125.9 °F; 185.5 K) | ||
| Solubility in water | 31.2 mg/100 ml (17 °C) | ||
| Viscosity | 1.1 x 10 Pa s | ||
| Structure | |||
| Molecular shape | Trigonal pyramidal | ||
| Dipole moment | 0.58 D | ||
| Thermochemistry | |||
| Std enthalpy of formation (ΔfH298) |
+22.89 kJ/mol | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | flammable gas | ||
| Explosive limits | 1.8% – ? | ||
| Related compounds | |||
| Other cations | Ammonia Arsine Stibine Bismuthine | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Phosphine (IUPAC name: phosphane) is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine (P2H4). With traces of P2H4 present, PH3 is spontaneously flammable in air, burning with a luminous flame. Phosphines are also a group of organophosphorus compounds with the formula R3P (R = organic derivative). Organophosphines are important in catalysts where they complex to various metal ions; complexes derived from a chiral phosphine can catalyze reactions to give chiral products.
History
Perhaps because of its strong association with elemental phosphorus, phosphine was once regarded as a gaseous form of the element but Lavoisier (1789) recognised it as a combination of phosphorus with hydrogen by describing it as “hydruyet of phosphorus, or phosphuret of hydrogen”.
Thénard (1845) used a cold trap to separate diphosphine from phosphine that had been generated from calcium phosphide, thereby demonstrating that P2H4 is responsible for spontaneous flammability associated with PH3, and also for the characteristic orange/brown colour that can form on surfaces, which is a polymerisation product. He considered diphosphine’s formula to be PH2, and thus an intermediate between elemental phosphorus, the higher polymers, and phosphine. Calcium phosphide (nominally Ca3P2) produces more P2H4 than other phosphides because of the preponderance of P-P bonds in the starting material.
Structure and properties
PH3 is a trigonal pyramidal molecule with C3v molecular symmetry. The length of the P-H bond 1.42 Å, the H-P-H bond angles are 93.5°. The dipole moment is 0.58 D, which increases with substitution of methyl groups in the series: CH3PH2, 1.10 D; (CH3)2PH, 1.23 D; (CH3)3P, 1.19 D. In contrast, the dipole moments of amines decrease with substitution, starting with ammonia, which has a dipole moment of 1.47 D. The low dipole moment and almost orthogonal bond angles lead to the conclusion that in PH3 the P-H bonds are almost entirely pσ(P) – sσ(H) and the lone pair contributes only a little to the molecular orbitals. The high positive chemical shift of the phosphorus atom in the P NMR spectrum accords with the conclusion that the lone pair electrons occupy the 3s orbital and so are close to the P atom (Fluck, 1973). This electronic structure leads to a lack of nucleophilicity and an inability to form hydrogen bonds.
The aqueous solubility of PH3 is slight; 0.22 mL of gas dissolve in 1 mL of water. Phosphine dissolves more readily in non-polar solvents than in water because of the non-polar P-H bonds. It acts as neither an acid nor a base in water. Proton exchange proceeds via a phosphonium (PH4) ion in acidic solutions and via PH2 at high pH, with equilibrium constants Kb = 4 × 10 and Kz = 41.6 × 10.
Preparation and occurrence
Phosphine may be prepared in a variety of ways. Industrially it can be made by the reaction of white phosphorus with sodium hydroxide, producing sodium hypophosphite and sodium phosphite as a by-product. Alternatively the acid-catalyzed disproportioning of white phosphorus may be used, which yields phosphoric acid and phosphine. Both routes have industrial significance; the acid route is preferred method if further reaction of the phosphine to substituted phosphines is needed. The acid route requires purification and pressurizing. It can also be made (as described above) by the hydrolysis of a metal phosphide such as aluminium phosphide or calcium phosphide. Pure samples of phosphine, free from P2H4, may be prepared using the action of potassium hydroxide on phosphonium iodide (PH4I).
Phosphine is probably a constituent of the atmosphere at very low and highly variable concentrations and hence may contribute to the global phosphorus biochemical cycle. The origin(s) of atmospheric phosphine is not certain. Possible sources include bacterial reduction of phosphate in decaying organic matter and the corrosion of phosphorus-containing metals.
Phosphines
Related to a PH3 is the class of organophosphorus compounds commonly called phosphines. These alkyl and aryl derivatives of phosphine are analogous to organic amines. Common examples include triphenylphosphine ((C6H5)3P) and BINAP, both used as ligands in homogeneous catalysis or triisopropylphosphine. Phosphines are easily oxidized to phosphine oxides as exemplified by the directed synthesis of a phospha-crown, the phosphorus analogue of an aza crown where it is not possible to isolate the phosphine itself.

In step 1 diphosphinoethane coordinates to a ferrocene containing additional carbon monoxide ligands and an acetonitrile ligand. The next step is a hydrophosphination with trivinylphosphine followed by alkylation with ethyl bromide and hydrogenation with hydrogen over palladium on carbon. In the final step the iron template is removed by bromine but oxidation of the phosphine groups is unavoidable.
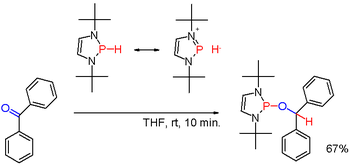
When modified with suitable substituents as in certain (rare) diazaphospholenes (scheme 3) the polarity of the P-H bond can be inverted (see: umpolung) and the resulting phosphine hydride can reduce a carbonyl group as in the example of benzophenone in yet another way.
Applications
Organophosphorus chemistry
Phosphine is mainly consumed as an intermediate in organophosphorus chemistry. In an illustrative reaction, formaldehyde adds in the presence of hydrogen chloride to give tetrakis(hydroxymethyl)phosphonium chloride, which is used in textiles.
Microelectronics
Small amounts of phosphine are used as a dopant in the semiconductor industry, and a precursor for the deposition of compound semiconductors.
Fumigant
For farm use, pellets of aluminium phosphide, calcium phosphide, or zinc phosphide release phosphine upon contact with atmospheric water or rodents' stomach acid. These pellets also contain agents to reduce the potential for ignition or explosion of the released phosphine.
Because the previously popular fumigant methyl bromide has been phased out in most countries under the Montreal Protocol, phosphine is the only widely used, cost effective, rapidly acting fumigant that does not leave residues on the stored product. Pests developing high levels of resistance toward phosphine have become common in Asia, Australia and Brazil. High level resistance is also likely to occur in other regions, but may not have been as closely monitored.
Safety
Phosphine gas may form explosive mixtures with air and can self ignite. The gas is heavier than air. When phosphine burns, it produces a dense white cloud of phosphorus pentoxide – a severe respiratory irritant.
Phosphine can be absorbed into the body by inhalation. Direct contact with phosphine liquid – although unlikely to occur – may cause frostbite. The main target organ of phosphine gas is the respiratory tract. According to the 2009 U.S. National Institute for Occupational Safety and Health (NIOSH) pocket guide, and U.S. Occupational Safety and Health Administration regulation the 8 hour average respiratory exposure should not exceed 0.3 ppm. NIOSH recommends that the short term respiratory exposure to phosphine gas should not exceed 1 ppm. The Immediately Dangerous to Life or Health level is 50 ppm. Overexposure to phosphine gas causes nausea, vomiting, abdominal pain, diarrhea; thirst; chest tightness, dyspnea (breathing difficulty); muscle pain, chills; stupor or syncope; pulmonary edema. Phosphine has been reported to have the odor of decaying fish at concentrations below 0.3 ppm. The smell normally is restricted to laboratory or processing phosphine as the smell comes from the way the phosphine is extracted from the environment. However, exposure to higher concentrations may cause olfactory fatigue.
See also
- Phosphine oxide, R3PO
- Phosphorane, R3PR2
- Phosphinite, R2(RO)P
- Phosphonite, R(RO)2P
- Phosphite, (RO)3P
- Phosphinate, R2P(RO)O
- Phosphonate, RP(RO)2O
- Phosphate, P(RO)3O
References
- A.D.F. Toy, The Chemistry of Phosphorus, Pergamon Press, Oxford, UK, 1973.
- Gassmann et al., "Phosphine in the lower terrestrial troposphere", Naturwissenschaften, 1996, 83(3), 129–31, (Eng).
- J. Roels & W. Verstraete, "Biological formation of volatile phosphorus compounds, a review paper", Bioresource Technology 79 (2001), 243–250.
- Edwards, P. G.; Haigh, R.; Li, D.; Newman, P. D. "Template Synthesis of 1,4,7-Triphosphacyclononanes." J. Am. Chem. Soc. 2006, 128, 3818–3830. doi:10.1021/ja0578956
- Burck, S.; Gudat, D.; Nieger, M.; Du Mont, W.-W. "P-Hydrogen-Substituted 1,3,2-Diazaphospholenes: Molecular Hydrides." J. Am. Chem. Soc. 2006, 128, 3946–3955. doi:10.1021/ja057827j
- Gerhard Bettermann, Werner Krause, Gerhard Riess, Thomas Hofmann “Phosphorus Compounds, Inorganic” in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a19_527
- "NIOSH Emergency Response Card". Cdc.gov. Retrieved 2010-04-06.
- "NIOSH pocket guide". Cdc.gov. 2009-02-03. Retrieved 2010-04-06.
- "WHO (Data Sheets on Pesticides-No. 46): Phosphine". Inchem.org. Retrieved 2010-04-06.
- "NIOSH Alert: Preventing Phosphine Poisoning and Explosions during Fumigation". Cdc.gov. 1995-07-10. Retrieved 2010-04-06.
- E. Fluck, The chemistry of phosphine, Topics in Current Chemistry Vol. 35, 64 pp, 1973.
- WHO (World Health Organisation), Phosphine and selected metal phosphides, Environmental Health Criteria. Published under the joint sponsorship of UNEP, ILO and WHO, Geneva, Vol. 73, 100 pp, 1988.
External links
| Phosphorus compounds | |
|---|---|
| Phosphides | |
| Other compounds | |

