| |

| |
| Names | |
|---|---|
| IUPAC name Cobalt(II) acetate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.000.687 |
| PubChem CID | |
| UNII |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Co(C2H3O2)2 |
| Molar mass | 177.02124 g/mol (anhydrous) 249.08 g/mol (tetrahydrate) |
| Appearance | Pink crystals (anhydrous) intense red crystals (tetrahydrate) |
| Odor | vinegar (tetrahydrate) |
| Density | 1.705 g/cm (tetrahydrate) |
| Melting point | 140 °C (284 °F; 413 K) (tetrahydrate) |
| Solubility in water | Soluble |
| Solubility | soluble in alcohol, dilute acids, pentyl acetate (tetrahydrate) |
| Magnetic susceptibility (χ) | +11,000·10 cm/mol |
| Refractive index (nD) | 1.542 (tetrahydrate) |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 503 mg/kg (oral, rat) |
| Safety data sheet (SDS) | J.T. Baker MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Cobalt(II) acetate is the cobalt salt of acetic acid. It is commonly found as the tetrahydrate Co(CH3CO2)2·4 H2O, abbreviated Co(OAc)2·4 H2O. It is used as a catalyst.
Synthesis and structure
Like many other transition metal acetates, cobalt(II) acetate forms by the reaction of cobalt oxide or hydroxide and acetic acid:
- CoO + 2 CH3CO2H + 3 H2O → Co(CH3CO2)2·4 H2O
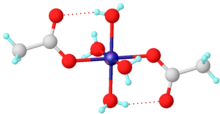
The tetrahydrate has been shown by X-ray crystallography to adopt an octahedral structure, the central cobalt centre being coordinated by four water molecules and two acetate ligands. The analogous nickel acetate is isostructural.
Various hydrates are known including Co(CH3CO2)2·H2O and 5·0.5 H2O.
Reactions and uses
Cobalt acetate is a precursor to various oil drying agents, catalysts that allow paints and varnishes to harden.
Anhydrous cobalt acetate is a widely used source of cobalt in the synthesis of materials, catalyst, and complexes.
Oxidation of acetic acid solutions of cobalt(II) acetate, e.g. with ozone, gives cobalt(III) acetates, which are strong oxidants.
Safety
Cobalt salts are poisonous.
References
- Sobolev, Alexandre N.; Miminoshvili, Elguja B.; Miminoshvili, Ketevan E.; Sakvarelidze, Tamara N. (2003). "Cobalt diacetate tetrahydrate". Acta Crystallographica Section E. 59 (10): m836 – m837. doi:10.1107/S1600536803019093.
- Van Niekerk, J. N.; Schoening, F. R. L. (1953). "The crystal structures of nickel acetate, Ni(CH3COO)2·4H2O, and cobalt acetate, Co(CH3COO)2·4H2O". Acta Crystallogr. 6 (7): 609–612. doi:10.1107/S0365110X5300171X.
- Zhang, Gao; Lin, Jian; Guo, Dong-Wei; Yao, Shi-Yan; Tian, Yun-Qi (2010). "Infinite Coordination Polymers of One- and Two-dimensional Cobalt Acetates". Zeitschrift für Anorganische und Allgemeine Chemie. 636 (7): 1401–1404. doi:10.1002/zaac.200900457.
- John Dallas Donaldson, Detmar Beyersmann, "Cobalt and Cobalt Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a07_281.pub2
- Rodenas, Tania; Luz, Ignacio; Prieto, Gonzalo; Seoane, Beatriz; Miro, Hozanna; Corma, Avelino; Kapteijn, Freek; Llabrés i Xamena, Francesc X.; Gascon, Jorge (2015). "Metal–organic framework nanosheets in polymer composite materials for gas separation". Nature Materials. 14 (1): 48–55. Bibcode:2015NatMa..14...48R. doi:10.1038/nmat4113. PMC 4270742. PMID 25362353.
- Schultz, Mitchell J.; Sigman, Matthew S. (2006). "Recent advances in homogeneous transition metal-catalyzed aerobic alcohol oxidations". Tetrahedron. 62 (35): 8227–8241. doi:10.1016/j.tet.2006.06.065.
- Appleton, T. G. (1977). "Oxygen Uptake by a Cobalt(II) Complex". J. Chem. Educ. 54 (7): 443. doi:10.1021/ed054p443.
- Periasamy, Mariappan; Radhakrishnan, Ukkiramapandian (2001). "Cobalt(III) Acetate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rc188. ISBN 0-471-93623-5.
| Cobalt compounds | |
|---|---|
| Cobalt(I) | |
| Cobalt(II) | |
| Cobalt(0,III) | |
| Cobalt(II,III) | |
| Cobalt(III) | |
| Cobalt(III,IV) | |
| Cobalt(IV) | |
| Cobalt(V) | |
| Acetyl halides and salts of the acetate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||