| Revision as of 09:54, 6 August 2011 editZéroBot (talk | contribs)704,777 editsm r2.7.1) (robot Adding: zh:溴化銫← Previous edit | Latest revision as of 20:49, 12 May 2024 edit undoCitation bot (talk | contribs)Bots5,433,404 edits Added bibcode. | Use this bot. Report bugs. | Suggested by Whoop whoop pull up | Category:Caesium compounds | #UCB_Category 8/47 | ||
| (64 intermediate revisions by 49 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 443321289 | ||
| ⚫ | | |
||
| | ImageFile = CsCl polyhedra.png | |||
| | ImageSize = |
| ImageSize = | ||
| ⚫ | | IUPACName = |
||
| ⚫ | | ImageFile1 = Caesium-bromide-3D-ionic.png | ||
| ⚫ | | IUPACName = Cesium bromide | ||
| | OtherNames = Cesium bromide,<br/>Caesium(I) bromide | | OtherNames = Cesium bromide,<br/>Caesium(I) bromide | ||
| | |
|Section1={{Chembox Identifiers | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChemSpiderID = 22994 | | ChemSpiderID = 22994 | ||
| | SMILES = . | | SMILES = . | ||
| | InChIKey = LYQFWZFBNBDLEO-REWHXWOFAA | | InChIKey = LYQFWZFBNBDLEO-REWHXWOFAA | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/BrH.Cs/h1H;/q;+1/p-1 | | StdInChI = 1S/BrH.Cs/h1H;/q;+1/p-1 | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = LYQFWZFBNBDLEO-UHFFFAOYSA-M | | StdInChIKey = LYQFWZFBNBDLEO-UHFFFAOYSA-M | ||
| | CASNo = 7787-69-1 | | CASNo = 7787-69-1 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | EINECS = 232-130-0 | |||
| | RTECS = FK9275000 | |||
| | UNII = 06M25EDM3F | |||
| ⚫ | |||
| | PubChem = 24592 | |||
| ⚫ | | InChI = 1/BrH.Cs/h1H;/q;+1/p-1 | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | Formula = CsBr | |||
| | MolarMass = 212.809 g/mol<ref name=r1>Haynes, p. 4.57</ref> | |||
| | Appearance = White solid | |||
| | Density = 4.43 g/cm<sup>3</sup><ref name=r1/> | |||
| | MeltingPtC = 636 | |||
| | MeltingPt_ref = <ref name=r1/> | |||
| | BoilingPt = 1300 °C | |||
| | BoilingPtC = 1300 | |||
| ⚫ | |||
| | BoilingPt_ref = <ref name=r1/> | |||
| ⚫ | | Solubility = 1230 g/L (25 °C)<ref name=r1/> Disputed. | ||
| 420 g/L (11 °C) See References<br/> 560 /L (15°C)<br/>1020 g/L (28.5 °C)<br/>1180 g/L (31 °C)<br/>1240 g/L (32.5 °C)<br/>1380 g/L (35 °C) | |||
| | RefractIndex = 1.8047 (0.3 μm)<br/>1.6974 (0.59 μm)<br/>1.6861 (0.75 μm)<br/>1.6784 (1 μm)<br/>1.6678 (5 μm)<br/>1.6439 (20 μm)<ref>Haynes, p. 10.240</ref> | |||
| | MagSus = -67.2·10<sup>−6</sup> cm<sup>3</sup>/mol<ref>Haynes, p. 4.132</ref> | |||
| }} | }} | ||
| | |
|Section3={{Chembox Structure | ||
| | |
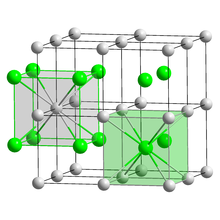
| CrystalStruct = ], ] | ||
| | SpaceGroup = Pm{{overline|3}}m, No. 221<ref name=str2>{{cite journal|doi=10.1063/1.1713597|title=Elastic Constants of CsBr and CsI from 4.2K to Room Temperature|journal=Journal of Applied Physics|volume=35|issue=4|pages=1222|year=1964|last1=Vallin|first1=J.|last2=Beckman|first2=O.|last3=Salama|first3=K.|bibcode=1964JAP....35.1222V}}</ref> | |||
| | Coordination = 8–8 | |||
| | LattConst_a = 0.4291 nm | |||
| | UnitCellFormulas = 1 | |||
| | UnitCellVolume =0.0790 nm<sup>3</sup> | |||
| | Coordination = Cubic (Cs<sup>+</sup>)<br/>Cubic (Br<sup>−</sup>) | |||
| }} | }} | ||
| | |
|Section4={{Chembox Hazards | ||
| | NFPA-H = 2 | |||
| | NFPA-F = 0 | |||
| | NFPA-R = 0 | |||
| | GHSSignalWord=Warning | |||
| ⚫ | |||
| | GHSPictograms={{GHS07}} | |||
| | HPhrases = {{H-phrases|302|315|319|335}} | |||
| | PPhrases = {{P-phrases|261|264|270|271|280|301+312|302+352|304+340|305+351+338|312|321|330|332+313|337+313|362|403+233|405|501}} | |||
| ⚫ | | FlashPt = Non-flammable | ||
| | LD50 = 1400 mg/kg (oral, rat)<ref>. nlm.nih.gov</ref> | |||
| }} | }} | ||
| | |
|Section8={{Chembox Related | ||
| | OtherAnions = ]<br/>]<br/>]<br/>] | |||
| | OtherCations = ]<br/>]<br/>]<br/>] | |||
| }} | }} | ||
| }} | }} | ||
| '''Caesium bromide''' |
'''Caesium bromide''' or '''cesium bromide''' is an ionic ] of ] and ] with the ] CsBr. It is a white or transparent solid with melting point at 636 °C that readily dissolves in water. Its bulk crystals have the cubic CsCl structure, but the structure changes to the ] type in nanometer-thin film grown on ], LiF, KBr or NaCl substrates.<ref name=str>{{cite journal|doi=10.1107/S0365110X51001641|title=Polymorphism of cesium and thallium halides|journal=Acta Crystallographica|volume=4|issue=6|pages=487–489|year=1951|last1=Schulz|first1=L. G.|bibcode=1951AcCry...4..487S }}</ref> | ||
| == Synthesis == | == Synthesis == | ||
| Caesium bromide can be prepared via following reactions: | |||
| * ]: | * ]: | ||
| : CsOH (aq) + HBr (aq) → CsBr (aq) + H<sub>2</sub>O (l) | : CsOH (aq) + HBr (aq) → CsBr (aq) + H<sub>2</sub>O (l) | ||
| : Cs<sub>2</sub>(CO<sub>3</sub>) (aq) + 2 HBr (aq) → 2 CsBr (aq) + H<sub>2</sub>O (l) + CO<sub>2</sub> (g) | : Cs<sub>2</sub>(CO<sub>3</sub>) (aq) + 2 HBr (aq) → 2 CsBr (aq) + H<sub>2</sub>O (l) + CO<sub>2</sub> (g) | ||
| * Direct synthesis: | * Direct synthesis: | ||
| : 2 Cs (s) + Br<sub>2</sub> (g) → 2 CsBr (s) | : 2 Cs (s) + Br<sub>2</sub> (g) → 2 CsBr (s) | ||
| The direct synthesis is a vigorous reaction of caesium with |
The direct synthesis is a vigorous reaction of caesium with bromine. Due to its high cost, it is not used for preparation. | ||
| == Uses == | == Uses == | ||
| Caesium bromide is sometimes used in optics as a ] component in wide-band ]. | Caesium bromide is sometimes used in optics as a ] component in wide-band ]. | ||
| == |
==References== | ||
| {{Reflist}}<br/>* {{Webarchive|url=https://web.archive.org/web/20121218002818/http://www.crystran.co.uk/caesium-bromide-csbr.htm |date=2012-12-18 }} | |||
| * ] | |||
| ⚫ | |||
| ==Cited sources== | |||
| * ] | |||
| *{{RubberBible92nd}} | |||
| == External links == | == External links == | ||
| {{Commons category|Caesium bromide}} | |||
| * | |||
| * {{Webarchive|url=https://web.archive.org/web/20071017044744/http://physchem.ox.ac.uk/MSDS/CA/caesium_bromide.html |date=2007-10-17 }} | |||
| * , , | |||
| * {{Webarchive|url=https://web.archive.org/web/20121218002818/http://www.crystran.co.uk/caesium-bromide-csbr.htm |date=2012-12-18 }}, | |||
| * | |||
| * | |||
| * | |||
| * | * | ||
| {{Caesium compounds}} | {{Caesium compounds}} | ||
| {{Bromides}} | |||
| {{Authority control}} | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 20:49, 12 May 2024
| |
| |
| Names | |
|---|---|
| IUPAC name Cesium bromide | |
| Other names
Cesium bromide, Caesium(I) bromide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.209 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | CsBr |
| Molar mass | 212.809 g/mol |
| Appearance | White solid |
| Density | 4.43 g/cm |
| Melting point | 636 °C (1,177 °F; 909 K) |
| Boiling point | 1,300 °C (2,370 °F; 1,570 K) |
| Solubility in water | 1230 g/L (25 °C) Disputed.
420 g/L (11 °C) See References |
| Magnetic susceptibility (χ) | -67.2·10 cm/mol |
| Refractive index (nD) | 1.8047 (0.3 μm) 1.6974 (0.59 μm) 1.6861 (0.75 μm) 1.6784 (1 μm) 1.6678 (5 μm) 1.6439 (20 μm) |
| Structure | |
| Crystal structure | CsCl, cP2 |
| Space group | Pm3m, No. 221 |
| Lattice constant | a = 0.4291 nm |
| Lattice volume (V) | 0.0790 nm |
| Formula units (Z) | 1 |
| Coordination geometry | Cubic (Cs) Cubic (Br) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 1400 mg/kg (oral, rat) |
| Related compounds | |
| Other anions | Caesium fluoride Caesium chloride Caesium iodide Caesium astatide |
| Other cations | Sodium bromide Potassium bromide Rubidium bromide Francium bromide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Caesium bromide or cesium bromide is an ionic compound of caesium and bromine with the chemical formula CsBr. It is a white or transparent solid with melting point at 636 °C that readily dissolves in water. Its bulk crystals have the cubic CsCl structure, but the structure changes to the rocksalt type in nanometer-thin film grown on mica, LiF, KBr or NaCl substrates.
Synthesis
Caesium bromide can be prepared via following reactions:
- CsOH (aq) + HBr (aq) → CsBr (aq) + H2O (l)
- Cs2(CO3) (aq) + 2 HBr (aq) → 2 CsBr (aq) + H2O (l) + CO2 (g)
- Direct synthesis:
- 2 Cs (s) + Br2 (g) → 2 CsBr (s)
The direct synthesis is a vigorous reaction of caesium with bromine. Due to its high cost, it is not used for preparation.
Uses
Caesium bromide is sometimes used in optics as a beamsplitter component in wide-band spectrophotometers.
References
- ^ Haynes, p. 4.57
- Haynes, p. 4.132
- Haynes, p. 10.240
- Vallin, J.; Beckman, O.; Salama, K. (1964). "Elastic Constants of CsBr and CsI from 4.2K to Room Temperature". Journal of Applied Physics. 35 (4): 1222. Bibcode:1964JAP....35.1222V. doi:10.1063/1.1713597.
- Caesium bromide. nlm.nih.gov
- Schulz, L. G. (1951). "Polymorphism of cesium and thallium halides". Acta Crystallographica. 4 (6): 487–489. Bibcode:1951AcCry...4..487S. doi:10.1107/S0365110X51001641.
* Crystran Ltd experimental data July 2021 Archived 2012-12-18 at the Wayback Machine
Cited sources
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, Florida: CRC Press. ISBN 1-4398-5511-0.
External links
- MSDS at Oxford University Archived 2007-10-17 at the Wayback Machine
- Crystran Physical data Archived 2012-12-18 at the Wayback Machine, IR transmission spectrum
- Ultra-violet photoabsorption measurements in alkali iodide and caesium bromide evaporated films
| Caesium compounds | |
|---|---|
| Salts and covalent derivatives of the bromide ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||