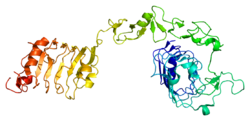| Revision as of 00:46, 29 November 2006 edit71.133.206.3 (talk)No edit summary← Previous edit | Latest revision as of 16:26, 25 May 2024 edit undoSimLibrarian (talk | contribs)Extended confirmed users124,530 editsm Updated short description #article-change-descTags: Mobile edit Mobile app edit iOS app edit | ||
| (183 intermediate revisions by 85 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Cell receptor protein found in humans}} | |||
| {{protein | |||
| {{cs1 config|name-list-style=vanc}} | |||
| | Name = insulin-like growth factor 1 receptor | |||
| {{Infobox_gene}} | |||
| | caption = | |||
| The '''insulin-like growth factor 1''' ('''IGF-1''') '''receptor''' is a ] found on the surface of human ]. It is a ] ] that is activated by a hormone called insulin-like growth factor 1 (]) and by a related hormone called ]. It belongs to the large class of ] receptors. This receptor mediates the effects of IGF-1, which is a polypeptide protein hormone similar in molecular structure to insulin. IGF-1 plays an important role in growth and continues to have anabolic effects in adults – meaning that it can induce hypertrophy of ] and other target tissues. Mice lacking the IGF-1 receptor die late in development, and show a dramatic reduction in body mass. This testifies to the strong growth-promoting effect of this receptor. | |||
| | image = | |||
| | width = | |||
| | HGNCid = 5465 | |||
| | Symbol = IGF1R | |||
| | AltSymbols = | |||
| | EntrezGene = 3480 | |||
| | OMIM = 147370 | |||
| | RefSeq = NM_000875 | |||
| | UniProt = P08069 | |||
| | PDB = | |||
| | ECnumber = | |||
| | Chromosome = 15 | |||
| | Arm = q | |||
| | Band = 26.3 | |||
| | LocusSupplementaryData = | |||
| }} | |||
| The Insulin-like Growth Factor 1 (]) Receptor is a ] ] that is activated by ] and by the related growth factor ]. It belongs to the large class of ] receptors. This receptor mediates the effects of ], which is a polypeptide protein hormone similar in molecular structure to insulin. IGF-1 plays an important role in growth and continues to have anabolic effects in adults - meaning that it can induce hypertrophy of ] and other target tissues. | |||
| == Structure == | |||
| ==Structure of receptor== | |||
| ] | |||
| Two alpha subunits and two beta subunits make up the '''IGF-1 receptor'''. The beta subunits pass through the cellular membrane and are linked by ]s. The receptor is a member of a family which consists of the ] and the ] (and their respective ligands IGF-1 and IGF2), along with several IGF-binding proteins. | |||
| Two alpha subunits and two beta subunits make up the IGF-1 receptor. Both the α and β subunits are synthesized from a single mRNA precursor. The precursor is then glycosylated, proteolytically cleaved, and crosslinked by cysteine bonds to form a functional transmembrane αβ chain.<ref name="Gregory">{{cite journal | vauthors = Gregory CW, DeGeorges A, Sikes RA | title = The IGF axis in the development and progression of prostate cancer | journal = Recent Research Developments in Cancer | pages = 437–462 | year = 2001 | isbn = 81-7895-002-2}}</ref> The α chains are located extracellularly, while the β subunit spans the membrane and is responsible for intracellular ] upon ligand stimulation. The mature IGF-1R has a molecular weight of approximately 320 kDa.<sup></sup> The receptor is a member of a family which consists of the ] and the ] (and their respective ligands IGF-1 and IGF-2), along with several IGF-binding proteins. | |||
| IGF-1R and IR both have a binding site for ], which is used to provide the phoshates for autophosphorylation (see below). There is a 60% homology between IGF-1R and the insulin receptor. | |||
| IGF-1R and the insulin receptor both have a binding site for ], which is used to provide the phosphates for ]. There is a 60% homology between IGF-1R and the insulin receptor. The structures of the autophosphorylation complexes of tyrosine residues 1165 and 1166 have been identified within crystals of the IGF1R kinase domain.<ref>{{cite journal | vauthors = Xu Q, Malecka KL, Fink L, Jordan EJ, Duffy E, Kolander S, Peterson JR, Dunbrack RL | title = Identifying three-dimensional structures of autophosphorylation complexes in crystals of protein kinases | journal = Science Signaling | volume = 8 | issue = 405 | pages = rs13 | date = December 2015 | pmid = 26628682 | pmc = 4766099 | doi = 10.1126/scisignal.aaa6711 }}</ref> | |||
| ==Receptor Family== | |||
| Tyrosine kinase receptors, including, the '''IGF-1 receptor''', mediate their activity by causing the addition of a ]s to particular tyrosines on certain ] within a cell. This addition of phosphate induces what are called "cell signaling" cascades - and the usual result of activation of the IGF-1 receptor is survival and proliferation in mitosis-competent cells, and growth (hypertrophy) in tissues such as ] and ]. | |||
| In response to ligand binding, the α chains induce the tyrosine autophosphorylation of the β chains. This event triggers a cascade of intracellular signaling that, while cell type-specific, often promotes cell survival and cell proliferation.<ref name="pmid7758431">{{cite journal | vauthors = Jones JI, Clemmons DR | title = Insulin-like growth factors and their binding proteins: biological actions | journal = Endocrine Reviews | volume = 16 | issue = 1 | pages = 3–34 | date = February 1995 | pmid = 7758431 | doi = 10.1210/edrv-16-1-3 }}</ref><ref name="pmid7540132">{{cite journal | vauthors = LeRoith D, Werner H, Beitner-Johnson D, Roberts CT | title = Molecular and cellular aspects of the insulin-like growth factor I receptor | journal = Endocrine Reviews | volume = 16 | issue = 2 | pages = 143–63 | date = April 1995 | pmid = 7540132 | doi = 10.1210/edrv-16-2-143 }}</ref> | |||
| The IGFR signalling pathway is of critical importance during normal development of mammary gland tissue during ] and ]. During pregnancy, there is intense ] of ] which form the duct and gland tissue. Following weaning, the cells undergo ] and all the tissue is destroyed. Several growth factors and hormones are involved in this overall process, and IGF-1R is believed to have roles in the differentiation of the cells and a key role in inhibiting apoptosis until weaning is complete. | |||
| ==Family members== | |||
| The IGF-1R is implicated in several cancers, most notably breast cancer. In some instances its anti-apoptotic properties allow cancerous cells to resist the ] properties of chemotheraputic drugs or radiotherapy. In others, where ] inhibitors such as ] are being used to inhibit the EGFR signalling pathway, IGF-1R confers resistance by forming one half of a heterodimer (see the description of EGFR signal transduction in the ] page), allowing EGFR signalling to resume in the presence of a suitable inhibitor. This process is referred to as crosstalk between EGFR and IGF-1R. | |||
| Tyrosine kinase receptors, including the IGF-1 receptor, mediate their activity by causing the addition of a ]s to particular tyrosines on certain ] within a cell. This addition of phosphate induces what are called "cell signaling" cascades - and the usual result of activation of the IGF-1 receptor is survival and proliferation in mitosis-competent cells, and growth (hypertrophy) in tissues such as ] and ]. | |||
| == Function == | |||
| It is further implicated in breast cancer by increasing the metastatic potential of the original tumour by inferring the ability to promote vascularisation. | |||
| === Embryonic development === | |||
| ==IGF-1 vs Insulin Receptor Signaling== | |||
| During embryonic development, the IGF-1R pathway is involved with the developing limb buds. | |||
| IGF-1 binds to at least two cell surface receptors: the IGF1 Receptor (IGFR), and the ]. The IGF-1 receptor seems to be the "physiologic" receptor - it binds IGF-1 at significantly higher affinity than it binds the insulin receptor. Like the insulin receptor, the IGF-1 receptor is a receptor tyrosine kinase - meaning it signals by causing the addition of a phosphate molecule on particular tyrosines. IGF-1 activates the Insulin receptor at approximately 0.1x the potency of insulin. Part of this signaling may be via IGF1R-InsulinReceptor heterodimers (the reason for the confusion is that binding studies show that IGF1 binds the insulin receptor 100-fold less well than insulin, yet that does not correlate with the actual potency of IGF1 in vivo at inducing phosphorylation of the Insulin receptor, and hypoglycemia). | |||
| == |
=== Lactation === | ||
| The IGFR signalling pathway is of critical importance during normal development of mammary gland tissue during ] and ]. During pregnancy, there is intense ] of ] which form the duct and gland tissue. Following weaning, the cells undergo ] and all the tissue is destroyed. Several growth factors and hormones are involved in this overall process, and IGF-1R is believed to have roles in the differentiation of the cells and a key role in inhibiting apoptosis until weaning is complete. | |||
| === Insulin signaling === | |||
| Due to the similarity of the structures of IGF-1R and the insulin receptor, especially in the regions of the ATP binding site and tyrosine kinase regions, synthesising selective inhibitors of IGF-1R is difficult. Prominent in current research are two main classes of inhibitor: | |||
| IGF-1 binds to at least two cell surface receptors: the IGF1 Receptor (IGFR), and the ]. The IGF-1 receptor seems to be the "physiologic" receptor—it binds IGF-1 at significantly higher affinity than it binds insulin.<ref>{{cite journal|vauthors=Hawsawi Y, El-Gendy R, Twelves C, Speirs V, Beattie J|date=December 2013|title=Insulin-like growth factor - oestradiol crosstalk and mammary gland tumourigenesis|journal=Biochimica et Biophysica Acta (BBA) - Reviews on Cancer|volume=1836|issue=2|pages=345–53|doi=10.1016/j.bbcan.2013.10.005|pmid=24189571|url=http://eprints.whiterose.ac.uk/80781/1/Insulin%20like%20growth%20factor%20Oestradiol%20crosstalk%20and%20mammary%20gland%20tumourigenesis.pdf}}</ref> Like the insulin receptor, the IGF-1 receptor is a receptor tyrosine kinase—meaning it signals by causing the addition of a phosphate molecule on particular tyrosines. IGF-1 activates the insulin receptor at approximately 10% the potency of insulin. Part of this signaling may be via IGF1R/insulin receptor heterodimers (the reason for the confusion is that binding studies show that IGF-1 binds the insulin receptor 100-fold less well than insulin, yet that does not correlate with the actual potency of IGF-1 ''in vivo'' at inducing phosphorylation of the insulin receptor, and hypoglycemia). | |||
| 1) Tyrphostins such as AG538 and AG1024. These are in early pre-clinical testing. They are not thought to be ATP-competative, although they are when used in EGFR as described in QSAR studies. These show some selectivity towards IGF-1R over IR. | |||
| 2) Pyrrolo-pyrimidine derivatives such as NVP-AEW541, which show far greater (100 fold) selectivity towards IFG-1R over IR. | |||
| === Aging === | |||
| Studies in female mice have shown that both ] (SON) and ] (PVN) lose approximately one-third of IGF-1R immunoreactive cells with normal aging. Also, old ] (CR) mice lost higher numbers of IGF-1R non-immunoreactive cells while maintaining similar counts of IGF-1R immunoreactive cells in comparison to old-Al mice. Consequently, old-CR mice show a higher percentage of IGF-1R immunoreactive cells, reflecting increased hypothalamic sensitivity to IGF-1 in comparison to normally aging mice.<ref name="pmid17194562">{{cite journal | vauthors = Saeed O, Yaghmaie F, Garan SA, Gouw AM, Voelker MA, Sternberg H, Timiras PS | title = Insulin-like growth factor-1 receptor immunoreactive cells are selectively maintained in the paraventricular hypothalamus of calorically restricted mice | journal = International Journal of Developmental Neuroscience | volume = 25 | issue = 1 | pages = 23–8 | date = February 2007 | pmid = 17194562 | doi = 10.1016/j.ijdevneu.2006.11.004 | s2cid = 5828689 | doi-access = free }}</ref><ref name="pmid17034982">{{cite journal | vauthors = Yaghmaie F, Saeed O, Garan SA, Voelker MA, Gouw AM, Freitag W, Sternberg H, Timiras PS | title = Age-dependent loss of insulin-like growth factor-1 receptor immunoreactive cells in the supraoptic hypothalamus is reduced in calorically restricted mice | journal = International Journal of Developmental Neuroscience | volume = 24 | issue = 7 | pages = 431–6 | date = November 2006 | pmid = 17034982 | doi = 10.1016/j.ijdevneu.2006.08.008 | s2cid = 22533403 }}</ref> | |||
| === Craniosynostosis === | |||
| == Effects of Aging on IGF-1R == | |||
| Mutations in IGF1R have been associated with ].<ref name="pmid21204214">{{cite journal | vauthors = Cunningham ML, Horst JA, Rieder MJ, Hing AV, Stanaway IB, Park SS, Samudrala R, Speltz ML | title = IGF1R variants associated with isolated single suture craniosynostosis | journal = American Journal of Medical Genetics. Part A | volume = 155A | issue = 1 | pages = 91–7 | date = January 2011 | pmid = 21204214 | pmc = 3059230 | doi = 10.1002/ajmg.a.33781 }}</ref> | |||
| Studies in female mice have shown that both Supraoptic nucleus (SON) and Paraventricular nucleus (PVN) lose about one-third of IGF-1R immunoreactive cells with normal aging. Also, Old caloricly restricted (CR) mice lost higher numbers of IGF-1R non-immunoreactive cells while maintaining similar counts of IGF-1R immunoreactive cells in comparison to Old-Al mice. Consequently, Old-CR mice show a higher percentage of IGF-1R immunoreactive cells reflecting increased hypothalamic sensitivity to IGF-1 in comparison to normally aging mice. | |||
| === Body size === | |||
| IGF-1R has been shown to have a significant effect on body size in small dog breeds.<ref name=":0" /> A "nonsynonymous SNP at chr3:44,706,389 that changes a highly conserved arginine at amino acid 204 to histidine" is associated with particularly tiny body size. "This mutation is predicted to prevent formation of several hydrogen bonds within the cysteine-rich domain of the receptor’s ligand-binding extracellular subunit. Nine of 13 tiny dog breeds carry the mutation and many dogs are homozygous for it." Smaller individuals within several small and medium-sized breeds were shown to carry this mutation as well. | |||
| Mice carrying only one functional copy of IGF-1R are normal, but exhibit a ~15% decrease in body mass. IGF-1R has also been shown to regulate body size in dogs. A mutated version of this gene is found in a number of small dog breeds.<ref name=":0">{{cite journal | vauthors = Hoopes BC, Rimbault M, Liebers D, Ostrander EA, Sutter NB | title = The insulin-like growth factor 1 receptor (IGF1R) contributes to reduced size in dogs | journal = Mammalian Genome | volume = 23 | issue = 11–12 | pages = 780–90 | date = December 2012 | pmid = 22903739 | pmc = 3511640 | doi = 10.1007/s00335-012-9417-z }}</ref> | |||
| ===Gene inactivation/deletion=== | |||
| == References == | |||
| Deletion of the IGF-1 receptor gene in mice results in lethality during early ]nic development, and for this reason, IGF-1 insensitivity, unlike the case of ] (GH) insensitivity (]), is not observed in the human population.<ref name="HarrisLippman2012">{{cite book| vauthors = Harris JR, Lippman ME, Osborne CK, Morrow M |title=Diseases of the Breast|url=https://books.google.com/books?id=GLc8xYe239kC&pg=PT88|date=28 March 2012|publisher=Lippincott Williams & Wilkins |isbn=978-1-4511-4870-1 |pages=88–}}</ref> | |||
| == Clinical significance == | |||
| * H.E. Jones, L. Goddard, J.M. Gee et al; ''Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells.'' Endocrine-Related Cancer,2004. '''11'''(4): p.793-814 | |||
| * Alexander Levitzki, Galia Blum, Aviv Gazit; ''Substrate Competitive Inhibitors of IGF-1 Receptor Kinase''; Biochemistry, 2000; '''39''': p.15705-15712 | |||
| * Warshamana-Greene, G.S., Litz, J., Buchdunger, E., et al; ''The Insulin-Like Growth Factor-I Receptor Kinase Inhibitor, NVP-ADW742, Sensitizes Small Cell Lung Cancer Cell Lines to the Effects of Chemotherapy, Clinical Cancer Research'', 2005; '''11''': p. 1563-1571 | |||
| == |
=== Cancer === | ||
| The IGF-1R is implicated in several cancers,<ref name="pmid15746061">{{cite journal | vauthors = Warshamana-Greene GS, Litz J, Buchdunger E, García-Echeverría C, Hofmann F, Krystal GW | title = The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy | journal = Clinical Cancer Research | volume = 11 | issue = 4 | pages = 1563–71 | date = February 2005 | pmid = 15746061 | doi = 10.1158/1078-0432.CCR-04-1544 | s2cid = 12090402 | doi-access = }}</ref><ref name="pmid15613453">{{cite journal | vauthors = Jones HE, Goddard L, Gee JM, Hiscox S, Rubini M, Barrow D, Knowlden JM, Williams S, Wakeling AE, Nicholson RI | title = Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells | journal = Endocrine-Related Cancer | volume = 11 | issue = 4 | pages = 793–814 | date = December 2004 | pmid = 15613453 | doi = 10.1677/erc.1.00799 | hdl = 11392/523178 | s2cid = 19466790 | doi-access = free }}</ref> including breast, prostate, and lung cancers. In some instances its anti-apoptotic properties allow cancerous cells to resist the ] properties of chemotherapeutic drugs or radiotherapy. In breast cancer, where ] inhibitors such as ] are being used to inhibit the EGFR signaling pathway, IGF-1R confers resistance by forming one half of a heterodimer (see the description of EGFR signal transduction in the ] page), allowing EGFR signaling to resume in the presence of a suitable inhibitor. This process is referred to as crosstalk between EGFR and IGF-1R. It is further implicated in breast cancer by increasing the metastatic potential of the original tumour by conferring the ability to promote vascularisation. | |||
| Increased levels of the IGF-IR are expressed in the majority of primary and metastatic prostate cancer patient tumors.<ref name="pmid12019176">{{cite journal | vauthors = Hellawell GO, Turner GD, Davies DR, Poulsom R, Brewster SF, Macaulay VM | title = Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease | journal = Cancer Research | volume = 62 | issue = 10 | pages = 2942–50 | date = May 2002 | pmid = 12019176 }}</ref> Evidence suggests that IGF-IR signaling is required for survival and growth when prostate cancer cells progress to androgen independence.<ref name="pmid15574769">{{cite journal | vauthors = Krueckl SL, Sikes RA, Edlund NM, Bell RH, Hurtado-Coll A, Fazli L, Gleave ME, Cox ME | title = Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model | journal = Cancer Research | volume = 64 | issue = 23 | pages = 8620–9 | date = December 2004 | pmid = 15574769 | doi = 10.1158/0008-5472.CAN-04-2446 | doi-access = free }}</ref> In addition, when immortalized prostate cancer cells mimicking advanced disease are treated with the IGF-1R ligand, IGF-1, the cells become more motile.<ref name="pmid16314838">{{cite journal | vauthors = Yao H, Dashner EJ, van Golen CM, van Golen KL | title = RhoC GTPase is required for PC-3 prostate cancer cell invasion but not motility | journal = Oncogene | volume = 25 | issue = 16 | pages = 2285–96 | date = April 2006 | pmid = 16314838 | doi = 10.1038/sj.onc.1209260 | doi-access = free }}</ref> | |||
| * ] | |||
| Members of the IGF receptor family and their ligands also seem to be involved in the carcinogenesis of mammary tumors of dogs.<ref name=Klopfleisch1>{{cite journal | vauthors = Klopfleisch R, Hvid H, Klose P, da Costa A, Gruber AD | title = Insulin receptor is expressed in normal canine mammary gland and benign adenomas but decreased in metastatic canine mammary carcinomas similar to human breast cancer | journal = Veterinary and Comparative Oncology | volume = 8 | issue = 4 | pages = 293–301 | date = December 2010 | pmid = 21062411 | doi = 10.1111/j.1476-5829.2009.00232.x | doi-access = }}</ref><ref name=Klopfleisch>{{cite journal | vauthors = Klopfleisch R, Lenze D, Hummel M, Gruber AD | title = Metastatic canine mammary carcinomas can be identified by a gene expression profile that partly overlaps with human breast cancer profiles | journal = BMC Cancer | volume = 10 | pages = 618 | date = November 2010 | pmid = 21062462 | pmc = 2994823 | doi = 10.1186/1471-2407-10-618 | doi-access = free }}</ref> IGF1R is amplified in several cancer types based on analysis of TCGA data, and gene amplification could be one mechanism for overexpression of IGF1R in cancer.<ref>{{cite journal | vauthors = Chen Y, McGee J, Chen X, Doman TN, Gong X, Zhang Y, Hamm N, Ma X, Higgs RE, Bhagwat SV, Buchanan S, Peng SB, Staschke KA, Yadav V, Yue Y, Kouros-Mehr H | title = Identification of druggable cancer driver genes amplified across TCGA datasets | journal = PLOS ONE | volume = 9 | issue = 5 | pages = e98293 | date = 2014 | pmid = 24874471 | pmc = 4038530 | doi = 10.1371/journal.pone.0098293 | bibcode = 2014PLoSO...998293C | doi-access = free }}</ref> | |||
| Lung cancer cells stimulated using ]s were induced into a reversible dormancy state which was dependent on the IGF-1R and its accompanying survival signaling pathways.<ref>{{cite journal| vauthors = Prekovic S, Schuurman K, Mayayo-Peralta I, Manjón AG, Buijs M, Yavuz S, Wellenstein MD, Barrera A, Monkhorst K, Huber A, Morris B|title=Glucocorticoid receptor triggers a reversible drug-tolerant dormancy state with acquired therapeutic vulnerabilities in lung cancer|journal=]|volume=12|issue=1|date=July 2021| page = 4360|doi = 10.1038/s41467-021-24537-3| pmid = 34272384| pmc = 8285479| bibcode = 2021NatCo..12.4360P| doi-access = free }}</ref> | |||
| ==Inhibitors== | |||
| Due to the similarity of the structures of IGF-1R and the insulin receptor (IR), especially in the regions of the ATP binding site and tyrosine kinase regions, synthesising selective inhibitors of IGF-1R is difficult. Prominent in current research are three main classes of inhibitor: | |||
| # ]s such as AG538<ref name="pmid11123895">{{cite journal | vauthors = Blum G, Gazit A, Levitzki A | title = Substrate competitive inhibitors of IGF-1 receptor kinase | journal = Biochemistry | volume = 39 | issue = 51 | pages = 15705–12 | date = December 2000 | pmid = 11123895 | doi = 10.1021/bi001516y }}</ref> and AG1024. These are in early pre-clinical testing. They are not thought to be ATP-competitive, although they are when used in EGFR as described in QSAR studies. These show some selectivity towards IGF-1R over IR. | |||
| # Pyrrolo(2,3-d)-pyrimidine derivatives such as NVP-AEW541, invented by Novartis, which show far greater (100 fold) selectivity towards IGF-1R over IR.<ref>{{Cite web |url=http://www.targeting-the-kinome.org/images/Garcia-Echeverria3.pdf |title=Archived copy |access-date=2012-07-18 |archive-date=2016-03-04 |archive-url=https://web.archive.org/web/20160304090810/http://www.targeting-the-kinome.org/images/Garcia-Echeverria3.pdf |url-status=dead }}</ref> | |||
| # ] are probably the most specific and promising therapeutic compounds. ] is a novel therapy showing significant benefit for ]. | |||
| == Interactions == | |||
| Insulin-like growth factor 1 receptor has been shown to ] with: | |||
| {{div col|colwidth=20em}} | |||
| * ],<ref name = pmid11724822>{{cite journal | vauthors = Taya S, Inagaki N, Sengiku H, Makino H, Iwamatsu A, Urakawa I, Nagao K, Kataoka S, Kaibuchi K | title = Direct interaction of insulin-like growth factor-1 receptor with leukemia-associated RhoGEF | journal = The Journal of Cell Biology | volume = 155 | issue = 5 | pages = 809–20 | date = November 2001 | pmid = 11724822 | pmc = 2150867 | doi = 10.1083/jcb.200106139 }}</ref> | |||
| * ],<ref name = pmid10026153>{{cite journal | vauthors = Arbet-Engels C, Tartare-Deckert S, Eckhart W | title = C-terminal Src kinase associates with ligand-stimulated insulin-like growth factor-I receptor | journal = The Journal of Biological Chemistry | volume = 274 | issue = 9 | pages = 5422–8 | date = February 1999 | pmid = 10026153 | doi = 10.1074/jbc.274.9.5422 | doi-access = free }}</ref> | |||
| * ],<ref name = pmid18632619>{{cite journal | vauthors = Sehat B, Andersson S, Girnita L, Larsson O | title = Identification of c-Cbl as a new ligase for insulin-like growth factor-I receptor with distinct roles from Mdm2 in receptor ubiquitination and endocytosis | journal = Cancer Research | volume = 68 | issue = 14 | pages = 5669–77 | date = July 2008 | pmid = 18632619 | doi = 10.1158/0008-5472.CAN-07-6364 | doi-access = }}</ref> | |||
| * ],<ref name = pmid11423532>{{cite journal | vauthors = Rotem-Yehudar R, Galperin E, Horowitz M | title = Association of insulin-like growth factor 1 receptor with EHD1 and SNAP29 | journal = The Journal of Biological Chemistry | volume = 276 | issue = 35 | pages = 33054–60 | date = August 2001 | pmid = 11423532 | doi = 10.1074/jbc.M009913200 | doi-access = free }}</ref> | |||
| * ],<ref name = pmid12697834>{{cite journal | vauthors = Vecchione A, Marchese A, Henry P, Rotin D, Morrione A | title = The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor | journal = Molecular and Cellular Biology | volume = 23 | issue = 9 | pages = 3363–72 | date = May 2003 | pmid = 12697834 | pmc = 153198 | doi = 10.1128/mcb.23.9.3363-3372.2003 }}</ref><ref name = pmid8776723>{{cite journal | vauthors = Dey BR, Frick K, Lopaczynski W, Nissley SP, Furlanetto RW | title = Evidence for the direct interaction of the insulin-like growth factor I receptor with IRS-1, Shc, and Grb10 | journal = Molecular Endocrinology | volume = 10 | issue = 6 | pages = 631–41 | date = June 1996 | pmid = 8776723 | doi = 10.1210/mend.10.6.8776723 | doi-access = free }}</ref><ref name = pmid9506989>{{cite journal | vauthors = He W, Rose DW, Olefsky JM, Gustafson TA | title = Grb10 interacts differentially with the insulin receptor, insulin-like growth factor I receptor, and epidermal growth factor receptor via the Grb10 Src homology 2 (SH2) domain and a second novel domain located between the pleckstrin homology and SH2 domains | journal = The Journal of Biological Chemistry | volume = 273 | issue = 12 | pages = 6860–7 | date = March 1998 | pmid = 9506989 | doi = 10.1074/jbc.273.12.6860 | doi-access = free }}</ref><ref name = pmid8764099>{{cite journal | vauthors = Morrione A, Valentinis B, Li S, Ooi JY, Margolis B, Baserga R | title = Grb10: A new substrate of the insulin-like growth factor I receptor | journal = Cancer Research | volume = 56 | issue = 14 | pages = 3165–7 | date = July 1996 | pmid = 8764099 }}</ref> | |||
| * ],<ref name = pmid8776723/><ref name = pmid10082579/><ref name = pmid7559507>{{cite journal | vauthors = Tartare-Deckert S, Sawka-Verhelle D, Murdaca J, Van Obberghen E | title = Evidence for a differential interaction of SHC and the insulin receptor substrate-1 (IRS-1) with the insulin-like growth factor-I (IGF-I) receptor in the yeast two-hybrid system | journal = The Journal of Biological Chemistry | volume = 270 | issue = 40 | pages = 23456–60 | date = October 1995 | pmid = 7559507 | doi = 10.1074/jbc.270.40.23456 | doi-access = free }}</ref> | |||
| * ],<ref name = pmid18632619/> | |||
| * ],<ref name = pmid18632619/><ref name = pmid12697834/> | |||
| * ],<ref name = pmid9415396>{{cite journal | vauthors = Mothe I, Delahaye L, Filloux C, Pons S, White MF, Van Obberghen E | title = Interaction of wild type and dominant-negative p55PIK regulatory subunit of phosphatidylinositol 3-kinase with insulin-like growth factor-1 signaling proteins | journal = Molecular Endocrinology | volume = 11 | issue = 13 | pages = 1911–23 | date = December 1997 | pmid = 9415396 | doi = 10.1210/mend.11.13.0029 | doi-access = free }}</ref> | |||
| * ],<ref name = pmid10082579>{{cite journal | vauthors = Mañes S, Mira E, Gómez-Mouton C, Zhao ZJ, Lacalle RA, Martínez-A C | title = Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility | journal = Molecular and Cellular Biology | volume = 19 | issue = 4 | pages = 3125–35 | date = April 1999 | pmid = 10082579 | pmc = 84106 | doi = 10.1128/mcb.19.4.3125 }}</ref><ref name = pmid7642582>{{cite journal | vauthors = Seely BL, Reichart DR, Staubs PA, Jhun BH, Hsu D, Maegawa H, Milarski KL, Saltiel AR, Olefsky JM | title = Localization of the insulin-like growth factor I receptor binding sites for the SH2 domain proteins p85, Syp, and GTPase activating protein | journal = The Journal of Biological Chemistry | volume = 270 | issue = 32 | pages = 19151–7 | date = August 1995 | pmid = 7642582 | doi = 10.1074/jbc.270.32.19151 | doi-access = free}}</ref> | |||
| * ],<ref name = pmid7642582/> | |||
| * ]<ref name = pmid8776723/><ref name = pmid7559507/><ref name = pmid16113100>{{cite journal | vauthors = Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura A, Lawrence J, Berstein L, Yue W | title = Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity | journal = Endocrine-Related Cancer | volume = 12 | pages = S61-73 | date = July 2005 | pmid = 16113100 | doi = 10.1677/erc.1.01018 | series = 12 | issue = Suppl 1 | s2cid = 18995886 }}</ref> | |||
| * ],<ref name = pmid9727029>{{cite journal | vauthors = Dey BR, Spence SL, Nissley P, Furlanetto RW | title = Interaction of human suppressor of cytokine signaling (SOCS)-2 with the insulin-like growth factor-I receptor | journal = The Journal of Biological Chemistry | volume = 273 | issue = 37 | pages = 24095–101 | date = September 1998 | pmid = 9727029 | doi = 10.1074/jbc.273.37.24095 | doi-access = free }}</ref> | |||
| * ],<ref name = pmid11071852>{{cite journal | vauthors = Dey BR, Furlanetto RW, Nissley P | title = Suppressor of cytokine signaling (SOCS)-3 protein interacts with the insulin-like growth factor-I receptor | journal = Biochemical and Biophysical Research Communications | volume = 278 | issue = 1 | pages = 38–43 | date = November 2000 | pmid = 11071852 | doi = 10.1006/bbrc.2000.3762 | url = https://zenodo.org/record/1229526 }}</ref> and | |||
| * ].<ref name = pmid9111084>{{cite journal | vauthors = Craparo A, Freund R, Gustafson TA | title = 14-3-3 (epsilon) interacts with the insulin-like growth factor I receptor and insulin receptor substrate I in a phosphoserine-dependent manner | journal = The Journal of Biological Chemistry | volume = 272 | issue = 17 | pages = 11663–9 | date = April 1997 | pmid = 9111084 | doi = 10.1074/jbc.272.17.11663 | doi-access = free }}</ref> | |||
| {{Div col end}} | |||
| ==Regulation== | |||
| There is evidence to suggest that IGF1R is negatively regulated by the ] ].<ref name="pmid20819078">{{cite journal | vauthors = Jiang L, Liu X, Chen Z, Jin Y, Heidbreder CE, Kolokythas A, Wang A, Dai Y, Zhou X | title = MicroRNA-7 targets IGF1R (insulin-like growth factor 1 receptor) in tongue squamous cell carcinoma cells | journal = The Biochemical Journal | volume = 432 | issue = 1 | pages = 199–205 | date = November 2010 | pmid = 20819078 | pmc = 3130335 | doi = 10.1042/BJ20100859 }}</ref> | |||
| == See also == | |||
| * ] | |||
| * ] | * ] | ||
| * ], an inhibitor of IGF-1R in clinical trials for cancer treatment | |||
| == References == | |||
| {{reflist|35em}} | |||
| == Further reading == | |||
| {{refbegin|35em}} | |||
| * {{cite journal | vauthors = Benito M, Valverde AM, Lorenzo M | title = IGF-I: a mitogen also involved in differentiation processes in mammalian cells | journal = The International Journal of Biochemistry & Cell Biology | volume = 28 | issue = 5 | pages = 499–510 | date = May 1996 | pmid = 8697095 | doi = 10.1016/1357-2725(95)00168-9 }} | |||
| * {{cite journal | vauthors = Butler AA, Yakar S, Gewolb IH, Karas M, Okubo Y, LeRoith D | title = Insulin-like growth factor-I receptor signal transduction: at the interface between physiology and cell biology | journal = Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology | volume = 121 | issue = 1 | pages = 19–26 | date = September 1998 | pmid = 9972281 | doi = 10.1016/S0305-0491(98)10106-2 | url = https://zenodo.org/record/1260019 }} | |||
| * {{cite journal | vauthors = Zhang X, Yee D | title = Tyrosine kinase signalling in breast cancer: insulin-like growth factors and their receptors in breast cancer | journal = Breast Cancer Research | volume = 2 | issue = 3 | pages = 170–5 | year = 2001 | pmid = 11250706 | pmc = 138771 | doi = 10.1186/bcr50 | doi-access = free }} | |||
| * {{cite journal | vauthors = Gross JM, Yee D | title = The type-1 insulin-like growth factor receptor tyrosine kinase and breast cancer: biology and therapeutic relevance | journal = Cancer and Metastasis Reviews | volume = 22 | issue = 4 | pages = 327–36 | date = December 2003 | pmid = 12884909 | doi = 10.1023/A:1023720928680 | s2cid = 35963688 }} | |||
| * {{cite journal | vauthors = Adams TE, McKern NM, Ward CW | title = Signalling by the type 1 insulin-like growth factor receptor: interplay with the epidermal growth factor receptor | journal = Growth Factors | volume = 22 | issue = 2 | pages = 89–95 | date = June 2004 | pmid = 15253384 | doi = 10.1080/08977190410001700998 | s2cid = 86844427 }} | |||
| * {{cite journal | vauthors = Surmacz E, Bartucci M | title = Role of estrogen receptor alpha in modulating IGF-I receptor signaling and function in breast cancer | journal = Journal of Experimental & Clinical Cancer Research | volume = 23 | issue = 3 | pages = 385–94 | date = September 2004 | pmid = 15595626 }} | |||
| * {{cite book | vauthors = Wood AW, Duan C, Bern HA | title = Insulin-like growth factor signaling in fish | volume = 243 | pages = 215–85 | year = 2005 | pmid = 15797461 | doi = 10.1016/S0074-7696(05)43004-1 | isbn = 978-0-12-364647-7 | series = International Review of Cytology }} | |||
| * {{cite journal | vauthors = Sarfstein R, Maor S, Reizner N, Abramovitch S, Werner H | title = Transcriptional regulation of the insulin-like growth factor-I receptor gene in breast cancer | journal = Molecular and Cellular Endocrinology | volume = 252 | issue = 1–2 | pages = 241–6 | date = June 2006 | pmid = 16647191 | doi = 10.1016/j.mce.2006.03.018 | s2cid = 24895685 | doi-access = free }} | |||
| {{refend}} | |||
| == External links == | == External links == | ||
| *{{MeshName|IGF-1+Receptor}} | |||
| * OMIM entry | |||
| * {{PDBe-KB2|P08069|Insulin-like growth factor 1 receptor}} | |||
| {{PDB Gallery|geneid=3480}} | |||
| ] | |||
| ] | |||
| ] | |||
| {{Clusters of differentiation}} | |||
| {{Growth factor receptors}} | |||
| {{Tyrosine kinases}} | |||
| {{Enzymes}} | |||
| {{Growth factor receptor modulators}} | |||
| {{Portal bar|Biology|border=no}} | |||
| ] | |||
| {{protein-stub}} | |||
| ] | |||
| ] | |||
Latest revision as of 16:26, 25 May 2024
Cell receptor protein found in humans
The insulin-like growth factor 1 (IGF-1) receptor is a protein found on the surface of human cells. It is a transmembrane receptor that is activated by a hormone called insulin-like growth factor 1 (IGF-1) and by a related hormone called IGF-2. It belongs to the large class of tyrosine kinase receptors. This receptor mediates the effects of IGF-1, which is a polypeptide protein hormone similar in molecular structure to insulin. IGF-1 plays an important role in growth and continues to have anabolic effects in adults – meaning that it can induce hypertrophy of skeletal muscle and other target tissues. Mice lacking the IGF-1 receptor die late in development, and show a dramatic reduction in body mass. This testifies to the strong growth-promoting effect of this receptor.
Structure

Two alpha subunits and two beta subunits make up the IGF-1 receptor. Both the α and β subunits are synthesized from a single mRNA precursor. The precursor is then glycosylated, proteolytically cleaved, and crosslinked by cysteine bonds to form a functional transmembrane αβ chain. The α chains are located extracellularly, while the β subunit spans the membrane and is responsible for intracellular signal transduction upon ligand stimulation. The mature IGF-1R has a molecular weight of approximately 320 kDa. The receptor is a member of a family which consists of the insulin receptor and the IGF-2R (and their respective ligands IGF-1 and IGF-2), along with several IGF-binding proteins.
IGF-1R and the insulin receptor both have a binding site for ATP, which is used to provide the phosphates for autophosphorylation. There is a 60% homology between IGF-1R and the insulin receptor. The structures of the autophosphorylation complexes of tyrosine residues 1165 and 1166 have been identified within crystals of the IGF1R kinase domain.
In response to ligand binding, the α chains induce the tyrosine autophosphorylation of the β chains. This event triggers a cascade of intracellular signaling that, while cell type-specific, often promotes cell survival and cell proliferation.
Family members
Tyrosine kinase receptors, including the IGF-1 receptor, mediate their activity by causing the addition of a phosphate groups to particular tyrosines on certain proteins within a cell. This addition of phosphate induces what are called "cell signaling" cascades - and the usual result of activation of the IGF-1 receptor is survival and proliferation in mitosis-competent cells, and growth (hypertrophy) in tissues such as skeletal muscle and cardiac muscle.
Function
Embryonic development
During embryonic development, the IGF-1R pathway is involved with the developing limb buds.
Lactation
The IGFR signalling pathway is of critical importance during normal development of mammary gland tissue during pregnancy and lactation. During pregnancy, there is intense proliferation of epithelial cells which form the duct and gland tissue. Following weaning, the cells undergo apoptosis and all the tissue is destroyed. Several growth factors and hormones are involved in this overall process, and IGF-1R is believed to have roles in the differentiation of the cells and a key role in inhibiting apoptosis until weaning is complete.
Insulin signaling
IGF-1 binds to at least two cell surface receptors: the IGF1 Receptor (IGFR), and the insulin receptor. The IGF-1 receptor seems to be the "physiologic" receptor—it binds IGF-1 at significantly higher affinity than it binds insulin. Like the insulin receptor, the IGF-1 receptor is a receptor tyrosine kinase—meaning it signals by causing the addition of a phosphate molecule on particular tyrosines. IGF-1 activates the insulin receptor at approximately 10% the potency of insulin. Part of this signaling may be via IGF1R/insulin receptor heterodimers (the reason for the confusion is that binding studies show that IGF-1 binds the insulin receptor 100-fold less well than insulin, yet that does not correlate with the actual potency of IGF-1 in vivo at inducing phosphorylation of the insulin receptor, and hypoglycemia).
Aging
Studies in female mice have shown that both supraoptic nucleus (SON) and paraventricular nucleus (PVN) lose approximately one-third of IGF-1R immunoreactive cells with normal aging. Also, old calorically restricted (CR) mice lost higher numbers of IGF-1R non-immunoreactive cells while maintaining similar counts of IGF-1R immunoreactive cells in comparison to old-Al mice. Consequently, old-CR mice show a higher percentage of IGF-1R immunoreactive cells, reflecting increased hypothalamic sensitivity to IGF-1 in comparison to normally aging mice.
Craniosynostosis
Mutations in IGF1R have been associated with craniosynostosis.
Body size
IGF-1R has been shown to have a significant effect on body size in small dog breeds. A "nonsynonymous SNP at chr3:44,706,389 that changes a highly conserved arginine at amino acid 204 to histidine" is associated with particularly tiny body size. "This mutation is predicted to prevent formation of several hydrogen bonds within the cysteine-rich domain of the receptor’s ligand-binding extracellular subunit. Nine of 13 tiny dog breeds carry the mutation and many dogs are homozygous for it." Smaller individuals within several small and medium-sized breeds were shown to carry this mutation as well.
Mice carrying only one functional copy of IGF-1R are normal, but exhibit a ~15% decrease in body mass. IGF-1R has also been shown to regulate body size in dogs. A mutated version of this gene is found in a number of small dog breeds.
Gene inactivation/deletion
Deletion of the IGF-1 receptor gene in mice results in lethality during early embryonic development, and for this reason, IGF-1 insensitivity, unlike the case of growth hormone (GH) insensitivity (Laron syndrome), is not observed in the human population.
Clinical significance
Cancer
The IGF-1R is implicated in several cancers, including breast, prostate, and lung cancers. In some instances its anti-apoptotic properties allow cancerous cells to resist the cytotoxic properties of chemotherapeutic drugs or radiotherapy. In breast cancer, where EGFR inhibitors such as erlotinib are being used to inhibit the EGFR signaling pathway, IGF-1R confers resistance by forming one half of a heterodimer (see the description of EGFR signal transduction in the erlotinib page), allowing EGFR signaling to resume in the presence of a suitable inhibitor. This process is referred to as crosstalk between EGFR and IGF-1R. It is further implicated in breast cancer by increasing the metastatic potential of the original tumour by conferring the ability to promote vascularisation.
Increased levels of the IGF-IR are expressed in the majority of primary and metastatic prostate cancer patient tumors. Evidence suggests that IGF-IR signaling is required for survival and growth when prostate cancer cells progress to androgen independence. In addition, when immortalized prostate cancer cells mimicking advanced disease are treated with the IGF-1R ligand, IGF-1, the cells become more motile. Members of the IGF receptor family and their ligands also seem to be involved in the carcinogenesis of mammary tumors of dogs. IGF1R is amplified in several cancer types based on analysis of TCGA data, and gene amplification could be one mechanism for overexpression of IGF1R in cancer.
Lung cancer cells stimulated using glucocorticoids were induced into a reversible dormancy state which was dependent on the IGF-1R and its accompanying survival signaling pathways.
Inhibitors
Due to the similarity of the structures of IGF-1R and the insulin receptor (IR), especially in the regions of the ATP binding site and tyrosine kinase regions, synthesising selective inhibitors of IGF-1R is difficult. Prominent in current research are three main classes of inhibitor:
- Tyrphostins such as AG538 and AG1024. These are in early pre-clinical testing. They are not thought to be ATP-competitive, although they are when used in EGFR as described in QSAR studies. These show some selectivity towards IGF-1R over IR.
- Pyrrolo(2,3-d)-pyrimidine derivatives such as NVP-AEW541, invented by Novartis, which show far greater (100 fold) selectivity towards IGF-1R over IR.
- Monoclonal antibodies are probably the most specific and promising therapeutic compounds. Teprotumumab is a novel therapy showing significant benefit for Thyroid Eye Disease.
Interactions
Insulin-like growth factor 1 receptor has been shown to interact with:
- ARHGEF12,
- C-src tyrosine kinase,
- Cbl gene,
- EHD1,
- GRB10,
- IRS1,
- Mdm2,
- NEDD4,
- PIK3R3,
- PTPN11,
- RAS p21 protein activator 1,
- SHC1
- SOCS2,
- SOCS3, and
- YWHAE.
Regulation
There is evidence to suggest that IGF1R is negatively regulated by the microRNA miR-7.
See also
- Hypothalamic–pituitary–somatic axis
- Insulin receptor
- Linsitinib, an inhibitor of IGF-1R in clinical trials for cancer treatment
References
- ^ GRCh38: Ensembl release 89: ENSG00000140443 – Ensembl, May 2017
- ^ GRCm38: Ensembl release 89: ENSMUSG00000005533 – Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Gregory CW, DeGeorges A, Sikes RA (2001). "The IGF axis in the development and progression of prostate cancer". Recent Research Developments in Cancer: 437–462. ISBN 81-7895-002-2.
- Xu Q, Malecka KL, Fink L, Jordan EJ, Duffy E, Kolander S, Peterson JR, Dunbrack RL (December 2015). "Identifying three-dimensional structures of autophosphorylation complexes in crystals of protein kinases". Science Signaling. 8 (405): rs13. doi:10.1126/scisignal.aaa6711. PMC 4766099. PMID 26628682.
- Jones JI, Clemmons DR (February 1995). "Insulin-like growth factors and their binding proteins: biological actions". Endocrine Reviews. 16 (1): 3–34. doi:10.1210/edrv-16-1-3. PMID 7758431.
- LeRoith D, Werner H, Beitner-Johnson D, Roberts CT (April 1995). "Molecular and cellular aspects of the insulin-like growth factor I receptor". Endocrine Reviews. 16 (2): 143–63. doi:10.1210/edrv-16-2-143. PMID 7540132.
- Hawsawi Y, El-Gendy R, Twelves C, Speirs V, Beattie J (December 2013). "Insulin-like growth factor - oestradiol crosstalk and mammary gland tumourigenesis" (PDF). Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 1836 (2): 345–53. doi:10.1016/j.bbcan.2013.10.005. PMID 24189571.
- Saeed O, Yaghmaie F, Garan SA, Gouw AM, Voelker MA, Sternberg H, Timiras PS (February 2007). "Insulin-like growth factor-1 receptor immunoreactive cells are selectively maintained in the paraventricular hypothalamus of calorically restricted mice". International Journal of Developmental Neuroscience. 25 (1): 23–8. doi:10.1016/j.ijdevneu.2006.11.004. PMID 17194562. S2CID 5828689.
- Yaghmaie F, Saeed O, Garan SA, Voelker MA, Gouw AM, Freitag W, Sternberg H, Timiras PS (November 2006). "Age-dependent loss of insulin-like growth factor-1 receptor immunoreactive cells in the supraoptic hypothalamus is reduced in calorically restricted mice". International Journal of Developmental Neuroscience. 24 (7): 431–6. doi:10.1016/j.ijdevneu.2006.08.008. PMID 17034982. S2CID 22533403.
- Cunningham ML, Horst JA, Rieder MJ, Hing AV, Stanaway IB, Park SS, Samudrala R, Speltz ML (January 2011). "IGF1R variants associated with isolated single suture craniosynostosis". American Journal of Medical Genetics. Part A. 155A (1): 91–7. doi:10.1002/ajmg.a.33781. PMC 3059230. PMID 21204214.
- ^ Hoopes BC, Rimbault M, Liebers D, Ostrander EA, Sutter NB (December 2012). "The insulin-like growth factor 1 receptor (IGF1R) contributes to reduced size in dogs". Mammalian Genome. 23 (11–12): 780–90. doi:10.1007/s00335-012-9417-z. PMC 3511640. PMID 22903739.
- Harris JR, Lippman ME, Osborne CK, Morrow M (28 March 2012). Diseases of the Breast. Lippincott Williams & Wilkins. pp. 88–. ISBN 978-1-4511-4870-1.
- Warshamana-Greene GS, Litz J, Buchdunger E, García-Echeverría C, Hofmann F, Krystal GW (February 2005). "The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy". Clinical Cancer Research. 11 (4): 1563–71. doi:10.1158/1078-0432.CCR-04-1544. PMID 15746061. S2CID 12090402.
- Jones HE, Goddard L, Gee JM, Hiscox S, Rubini M, Barrow D, Knowlden JM, Williams S, Wakeling AE, Nicholson RI (December 2004). "Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells". Endocrine-Related Cancer. 11 (4): 793–814. doi:10.1677/erc.1.00799. hdl:11392/523178. PMID 15613453. S2CID 19466790.
- Hellawell GO, Turner GD, Davies DR, Poulsom R, Brewster SF, Macaulay VM (May 2002). "Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease". Cancer Research. 62 (10): 2942–50. PMID 12019176.
- Krueckl SL, Sikes RA, Edlund NM, Bell RH, Hurtado-Coll A, Fazli L, Gleave ME, Cox ME (December 2004). "Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model". Cancer Research. 64 (23): 8620–9. doi:10.1158/0008-5472.CAN-04-2446. PMID 15574769.
- Yao H, Dashner EJ, van Golen CM, van Golen KL (April 2006). "RhoC GTPase is required for PC-3 prostate cancer cell invasion but not motility". Oncogene. 25 (16): 2285–96. doi:10.1038/sj.onc.1209260. PMID 16314838.
- Klopfleisch R, Hvid H, Klose P, da Costa A, Gruber AD (December 2010). "Insulin receptor is expressed in normal canine mammary gland and benign adenomas but decreased in metastatic canine mammary carcinomas similar to human breast cancer". Veterinary and Comparative Oncology. 8 (4): 293–301. doi:10.1111/j.1476-5829.2009.00232.x. PMID 21062411.
- Klopfleisch R, Lenze D, Hummel M, Gruber AD (November 2010). "Metastatic canine mammary carcinomas can be identified by a gene expression profile that partly overlaps with human breast cancer profiles". BMC Cancer. 10: 618. doi:10.1186/1471-2407-10-618. PMC 2994823. PMID 21062462.
- Chen Y, McGee J, Chen X, Doman TN, Gong X, Zhang Y, Hamm N, Ma X, Higgs RE, Bhagwat SV, Buchanan S, Peng SB, Staschke KA, Yadav V, Yue Y, Kouros-Mehr H (2014). "Identification of druggable cancer driver genes amplified across TCGA datasets". PLOS ONE. 9 (5): e98293. Bibcode:2014PLoSO...998293C. doi:10.1371/journal.pone.0098293. PMC 4038530. PMID 24874471.
- Prekovic S, Schuurman K, Mayayo-Peralta I, Manjón AG, Buijs M, Yavuz S, Wellenstein MD, Barrera A, Monkhorst K, Huber A, Morris B (July 2021). "Glucocorticoid receptor triggers a reversible drug-tolerant dormancy state with acquired therapeutic vulnerabilities in lung cancer". Nature Communications. 12 (1): 4360. Bibcode:2021NatCo..12.4360P. doi:10.1038/s41467-021-24537-3. PMC 8285479. PMID 34272384.
- Blum G, Gazit A, Levitzki A (December 2000). "Substrate competitive inhibitors of IGF-1 receptor kinase". Biochemistry. 39 (51): 15705–12. doi:10.1021/bi001516y. PMID 11123895.
- "Archived copy" (PDF). Archived from the original (PDF) on 2016-03-04. Retrieved 2012-07-18.
{{cite web}}: CS1 maint: archived copy as title (link) - Taya S, Inagaki N, Sengiku H, Makino H, Iwamatsu A, Urakawa I, Nagao K, Kataoka S, Kaibuchi K (November 2001). "Direct interaction of insulin-like growth factor-1 receptor with leukemia-associated RhoGEF". The Journal of Cell Biology. 155 (5): 809–20. doi:10.1083/jcb.200106139. PMC 2150867. PMID 11724822.
- Arbet-Engels C, Tartare-Deckert S, Eckhart W (February 1999). "C-terminal Src kinase associates with ligand-stimulated insulin-like growth factor-I receptor". The Journal of Biological Chemistry. 274 (9): 5422–8. doi:10.1074/jbc.274.9.5422. PMID 10026153.
- ^ Sehat B, Andersson S, Girnita L, Larsson O (July 2008). "Identification of c-Cbl as a new ligase for insulin-like growth factor-I receptor with distinct roles from Mdm2 in receptor ubiquitination and endocytosis". Cancer Research. 68 (14): 5669–77. doi:10.1158/0008-5472.CAN-07-6364. PMID 18632619.
- Rotem-Yehudar R, Galperin E, Horowitz M (August 2001). "Association of insulin-like growth factor 1 receptor with EHD1 and SNAP29". The Journal of Biological Chemistry. 276 (35): 33054–60. doi:10.1074/jbc.M009913200. PMID 11423532.
- ^ Vecchione A, Marchese A, Henry P, Rotin D, Morrione A (May 2003). "The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor". Molecular and Cellular Biology. 23 (9): 3363–72. doi:10.1128/mcb.23.9.3363-3372.2003. PMC 153198. PMID 12697834.
- ^ Dey BR, Frick K, Lopaczynski W, Nissley SP, Furlanetto RW (June 1996). "Evidence for the direct interaction of the insulin-like growth factor I receptor with IRS-1, Shc, and Grb10". Molecular Endocrinology. 10 (6): 631–41. doi:10.1210/mend.10.6.8776723. PMID 8776723.
- He W, Rose DW, Olefsky JM, Gustafson TA (March 1998). "Grb10 interacts differentially with the insulin receptor, insulin-like growth factor I receptor, and epidermal growth factor receptor via the Grb10 Src homology 2 (SH2) domain and a second novel domain located between the pleckstrin homology and SH2 domains". The Journal of Biological Chemistry. 273 (12): 6860–7. doi:10.1074/jbc.273.12.6860. PMID 9506989.
- Morrione A, Valentinis B, Li S, Ooi JY, Margolis B, Baserga R (July 1996). "Grb10: A new substrate of the insulin-like growth factor I receptor". Cancer Research. 56 (14): 3165–7. PMID 8764099.
- ^ Mañes S, Mira E, Gómez-Mouton C, Zhao ZJ, Lacalle RA, Martínez-A C (April 1999). "Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility". Molecular and Cellular Biology. 19 (4): 3125–35. doi:10.1128/mcb.19.4.3125. PMC 84106. PMID 10082579.
- ^ Tartare-Deckert S, Sawka-Verhelle D, Murdaca J, Van Obberghen E (October 1995). "Evidence for a differential interaction of SHC and the insulin receptor substrate-1 (IRS-1) with the insulin-like growth factor-I (IGF-I) receptor in the yeast two-hybrid system". The Journal of Biological Chemistry. 270 (40): 23456–60. doi:10.1074/jbc.270.40.23456. PMID 7559507.
- Mothe I, Delahaye L, Filloux C, Pons S, White MF, Van Obberghen E (December 1997). "Interaction of wild type and dominant-negative p55PIK regulatory subunit of phosphatidylinositol 3-kinase with insulin-like growth factor-1 signaling proteins". Molecular Endocrinology. 11 (13): 1911–23. doi:10.1210/mend.11.13.0029. PMID 9415396.
- ^ Seely BL, Reichart DR, Staubs PA, Jhun BH, Hsu D, Maegawa H, Milarski KL, Saltiel AR, Olefsky JM (August 1995). "Localization of the insulin-like growth factor I receptor binding sites for the SH2 domain proteins p85, Syp, and GTPase activating protein". The Journal of Biological Chemistry. 270 (32): 19151–7. doi:10.1074/jbc.270.32.19151. PMID 7642582.
- Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura A, Lawrence J, Berstein L, Yue W (July 2005). "Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity". Endocrine-Related Cancer. 12. 12 (Suppl 1): S61-73. doi:10.1677/erc.1.01018. PMID 16113100. S2CID 18995886.
- Dey BR, Spence SL, Nissley P, Furlanetto RW (September 1998). "Interaction of human suppressor of cytokine signaling (SOCS)-2 with the insulin-like growth factor-I receptor". The Journal of Biological Chemistry. 273 (37): 24095–101. doi:10.1074/jbc.273.37.24095. PMID 9727029.
- Dey BR, Furlanetto RW, Nissley P (November 2000). "Suppressor of cytokine signaling (SOCS)-3 protein interacts with the insulin-like growth factor-I receptor". Biochemical and Biophysical Research Communications. 278 (1): 38–43. doi:10.1006/bbrc.2000.3762. PMID 11071852.
- Craparo A, Freund R, Gustafson TA (April 1997). "14-3-3 (epsilon) interacts with the insulin-like growth factor I receptor and insulin receptor substrate I in a phosphoserine-dependent manner". The Journal of Biological Chemistry. 272 (17): 11663–9. doi:10.1074/jbc.272.17.11663. PMID 9111084.
- Jiang L, Liu X, Chen Z, Jin Y, Heidbreder CE, Kolokythas A, Wang A, Dai Y, Zhou X (November 2010). "MicroRNA-7 targets IGF1R (insulin-like growth factor 1 receptor) in tongue squamous cell carcinoma cells". The Biochemical Journal. 432 (1): 199–205. doi:10.1042/BJ20100859. PMC 3130335. PMID 20819078.
Further reading
- Benito M, Valverde AM, Lorenzo M (May 1996). "IGF-I: a mitogen also involved in differentiation processes in mammalian cells". The International Journal of Biochemistry & Cell Biology. 28 (5): 499–510. doi:10.1016/1357-2725(95)00168-9. PMID 8697095.
- Butler AA, Yakar S, Gewolb IH, Karas M, Okubo Y, LeRoith D (September 1998). "Insulin-like growth factor-I receptor signal transduction: at the interface between physiology and cell biology". Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 121 (1): 19–26. doi:10.1016/S0305-0491(98)10106-2. PMID 9972281.
- Zhang X, Yee D (2001). "Tyrosine kinase signalling in breast cancer: insulin-like growth factors and their receptors in breast cancer". Breast Cancer Research. 2 (3): 170–5. doi:10.1186/bcr50. PMC 138771. PMID 11250706.
- Gross JM, Yee D (December 2003). "The type-1 insulin-like growth factor receptor tyrosine kinase and breast cancer: biology and therapeutic relevance". Cancer and Metastasis Reviews. 22 (4): 327–36. doi:10.1023/A:1023720928680. PMID 12884909. S2CID 35963688.
- Adams TE, McKern NM, Ward CW (June 2004). "Signalling by the type 1 insulin-like growth factor receptor: interplay with the epidermal growth factor receptor". Growth Factors. 22 (2): 89–95. doi:10.1080/08977190410001700998. PMID 15253384. S2CID 86844427.
- Surmacz E, Bartucci M (September 2004). "Role of estrogen receptor alpha in modulating IGF-I receptor signaling and function in breast cancer". Journal of Experimental & Clinical Cancer Research. 23 (3): 385–94. PMID 15595626.
- Wood AW, Duan C, Bern HA (2005). Insulin-like growth factor signaling in fish. International Review of Cytology. Vol. 243. pp. 215–85. doi:10.1016/S0074-7696(05)43004-1. ISBN 978-0-12-364647-7. PMID 15797461.
- Sarfstein R, Maor S, Reizner N, Abramovitch S, Werner H (June 2006). "Transcriptional regulation of the insulin-like growth factor-I receptor gene in breast cancer". Molecular and Cellular Endocrinology. 252 (1–2): 241–6. doi:10.1016/j.mce.2006.03.018. PMID 16647191. S2CID 24895685.
External links
- IGF-1+Receptor at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P08069 (Insulin-like growth factor 1 receptor) at the PDBe-KB.
| PDB gallery | |
|---|---|
|
| Proteins: clusters of differentiation (see also list of human clusters of differentiation) | |
|---|---|
| 1–50 | |
| 51–100 | |
| 101–150 | |
| 151–200 | |
| 201–250 | |
| 251–300 | |
| 301–350 | |
| Receptors: growth factor receptors | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I cytokine receptor | |||||||||||||
| Receptor protein serine/threonine kinase | |||||||||||||
| Receptor tyrosine kinase |
| ||||||||||||
| Tumor necrosis factor receptor | |||||||||||||
| Ig superfamily | |||||||||||||
| Other/ungrouped | |||||||||||||
| Protein kinases: tyrosine kinases (EC 2.7.10) | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||
| Enzymes | |
|---|---|
| Activity | |
| Regulation | |
| Classification | |
| Kinetics | |
| Types |
|
| Growth factor receptor modulators | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiopoietin |
| ||||||||||
| CNTF |
| ||||||||||
| EGF (ErbB) |
| ||||||||||
| FGF |
| ||||||||||
| HGF (c-Met) |
| ||||||||||
| IGF |
| ||||||||||
| LNGF (p75) |
| ||||||||||
| PDGF |
| ||||||||||
| RET (GFL) |
| ||||||||||
| SCF (c-Kit) |
| ||||||||||
| TGFβ |
| ||||||||||
| Trk |
| ||||||||||
| VEGF |
| ||||||||||
| Others |
| ||||||||||