
| |
| |
| Identifiers | |
|---|---|
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.246 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula |
|
| Molar mass | 184.113 g/mol (anhydrous) 292.204 g/mol (hexahydrate) |
| Appearance | white hygroscopic hexagonal crystals (anhydrous) colorless monoclinic crystals (hexahydrate) |
| Density | 3.72 g/cm (anhydrous) 2.07 g/cm (hexahydrate) |
| Melting point | 711 °C (1,312 °F; 984 K) 172.4 °C, decomposes (hexahydrate) |
| Boiling point | 1,250 °C (2,280 °F; 1,520 K) |
| Solubility in water | 102 g/(100 mL) (anhydrous) 316 g/(100 mL) (0 °C, hexahydrate) |
| Solubility | ethanol: 6.9 g/(100 mL) methanol: 21.8 g/(100 mL) |
| Magnetic susceptibility (χ) | −72.0·10 cm/mol |
| Structure | |
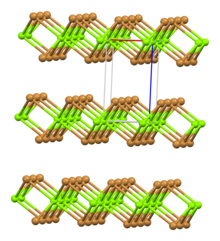
| Crystal structure | Rhombohedral, hP3 |
| Space group | P-3m1, No. 164 |
| Coordination geometry | octahedral |
| Thermochemistry | |
| Heat capacity (C) | 70 J/(mol·K) |
| Std molar entropy (S298) |
117.2 J/(mol·K) |
| Std enthalpy of formation (ΔfH298) |
−524.3 kJ/mol |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Safety data sheet (SDS) | External SDS |
| Related compounds | |
| Other anions | |
| Other cations | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Magnesium bromide are inorganic compounds with the chemical formula MgBr2(H2O)x, where x can range from 0 to 9. They are all white deliquescent solids. Some magnesium bromides have been found naturally as rare minerals such as: bischofite and carnallite.
Synthesis
Magnesium bromide can be synthesized by treating magnesium oxide (and related basic salts) with hydrobromic acid. It can also be made by reacting magnesium carbonate and hydrobromic acids, and collecting the solid left after evaporation.
As suggested by its easy conversion to various hydrates, anhydrous MgBr2 is a Lewis acid. In the coordination polymer with the formula MgBr2(dioxane)2, Mg adopts an octahedral geometry.
Uses and reactions
Magnesium bromide is used as a Lewis acid catalyst in some organic synthesis, e.g., in aldol reaction.
Magnesium bromide also has been used as a tranquilizer and as an anticonvulsant for treatment of nervous disorders.
Magnesium bromide modifies the catalytic properties of palladium on charcoal.
Magnesium bromide hexahydrate has properties as a flame retardant.
Treatment of magnesium bromide with chlorine gives magnesium chloride. This reaction is employed in the production of magnesium chloride from brines.
Structure
Two hydrates are known, the hexahydrate and the nonahydrate. Several reports claim a decahydrate, but X-ray crystallography confirmed that it is a nonahydrate. The hydrates feature ions.
References
- Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 4–67. ISBN 0-8493-0594-2.
- ^ Gruyter, W. Concise Encyclopedia Chemistry, Walter de Gruyter & Company: Berlin, 1993; 612
- ^ Lewis, R.J. Hawley’s Condensed Chemical Dictionary, 15th ed.; John Wiley & Sons Inc.:New York, 2007; 777
- Fischer, Reinald; Görls, Helmar; Meisinger, Philippe R.; Suxdorf, Regina; Westerhausen, Matthias (2019). "Structure–Solubility Relationship of 1,4-Dioxane Complexes of Di(hydrocarbyl)magnesium". Chemistry – A European Journal. 25 (55): 12830–12841. doi:10.1002/chem.201903120. PMC 7027550. PMID 31328293.
- Evans, David A.; Tedrow, Jason S.; Shaw, Jared T.; Downey, C. Wade (2002). "Diastereoselective Magnesium Halide-Catalyzed anti-Aldol Reactions of Chiral N-Acyloxazolidinones". Journal of the American Chemical Society. 124 (3): 392–393. doi:10.1021/ja0119548. PMID 11792206.
- Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- Bouzide, Abderrahim (2002). "Magnesium Bromide Mediated Highly Diastereoselective Heterogeneous Hydrogenation of Olefins". Organic Letters. 4 (8): 1347–50. doi:10.1021/ol020032m. PMID 11950359.
- Mostashari, S. M.; Fayyaz, F. (2008). "XRD characterization of the ashes from a burned cellulosic fabric impregnated with magnesium bromide hexahydrate as flame-retardant". Journal of Thermal Analysis and Calorimetry. 92 (3): 845. doi:10.1007/s10973-007-8928-4. S2CID 94416902.
- Seeger, Margarete; Otto, Walter; Flick, Wilhelm; Bickelhaupt, Friedrich; Akkerman, Otto S. (2000). "Magnesium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a15_595. ISBN 3-527-30673-0.
- Hennings, Erik; Schmidt, Horst; Voigt, Wolfgang (2013). "Crystal Structures of Hydrates of Simple Inorganic Salts. I. Water-Rich Magnesium Halide Hydrates MgCl2·8H2O, MgCl2·12H2O, MgBr2·6H2O, MgBr2·9H2O, MgI2·8H2O and MgI2·9H2O". Acta Crystallographica Section C Crystal Structure Communications. 69 (11): 1292–1300. doi:10.1107/S0108270113028138. PMID 24192174.
| Magnesium compounds | |
|---|---|
| Salts and covalent derivatives of the bromide ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bromine compounds | |
|---|---|
| Br(−I) | |
| Br(−I,I) | |
| Br(I) | |
| Br(II) | |
| Br(I,V) | |
| Br(III) | |
| Br(IV) | |
| Br(V) | |
| Br(VII) | |