| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Codeine/paracetamol" – news · newspapers · books · scholar · JSTOR (July 2012) (Learn how and when to remove this message) |
Pharmaceutical compound
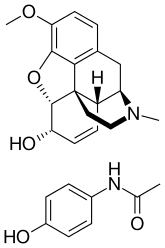 | |
| Combination of | |
|---|---|
| Codeine | Opioid analgesic |
| paracetamol | Anilide analgesic |
| Clinical data | |
| Trade names | Tylenol with codeine, others |
| MedlinePlus | a601005 |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| (what is this?) (verify) | |
Codeine/paracetamol, also called codeine/acetaminophen and co-codamol, is a compound analgesic, comprising codeine phosphate and paracetamol (acetaminophen). Codeine/paracetamol is used for the relief of mild to moderate pain when paracetamol or non-steroidal anti-inflammatory drugs (NSAIDs; such as ibuprofen, aspirin, and naproxen) alone do not sufficiently relieve symptoms.
In 2022, it was the 166th most commonly prescribed medication in the United States, with more than 3 million prescriptions.
Side effects
The most common side effects include constipation, nausea and drowsiness. Others include coughing up blood from the lungs, skin rashes, dizziness, sedation, shortness of breath, hypersensitivity reaction, fainting (syncope or near syncope), confusion, loss of short-term memory, changes in blood, allergic reactions, euphoria, dysphoria, abdominal pain, itchiness, easy bruising, bleeding gums, vivid dreams, dry mouth and addiction.
Genetic differences between people cause differing rates of metabolism of codeine to morphine. In about 5 percent of people this may happen particularly fast, causing morphine to be passed through breast milk in amounts that may cause fatal respiratory depression in a breastfed baby.
Society and culture
Availability
Of the European Union member states, eleven allow over-the-counter sale of solid dosage forms of codeine, including codeine/paracetamol: Bulgaria, Cyprus, Denmark, France, Ireland, Latvia, Lithuania, Malta, Poland, Romania, and Slovenia.
References
- "Acetaminophen with Codeine Product information". Health Canada. 25 April 2012. Archived from the original on 13 September 2022. Retrieved 13 September 2022.
- ^ "Boots Paracetamol & Codeine 500mg/8mg Tablets - Patient Information Leaflet (PIL)". (emc). 30 November 2021. Archived from the original on 18 July 2022. Retrieved 17 July 2022.
- ^ "Co-Codamol 15/500 Tablets - Summary of Product Characteristics (SmPC)". (emc). 25 March 2021. Archived from the original on 5 August 2021. Retrieved 17 July 2022.
- "Controlled Substances - Alphabetical Order" (PDF). Archived (PDF) from the original on 21 April 2021. Retrieved 29 June 2024.
- "Acetaminophen and Codeine Phosphate tablet". DailyMed. 31 July 2020. Archived from the original on 18 March 2022. Retrieved 17 July 2022.
- "Acetaminophen and Codeine Phosphate solution". DailyMed. 25 May 2022. Archived from the original on 11 May 2021. Retrieved 17 July 2022.
- "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- "Acetaminophen; Codeine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- "Co-codamol for adults: painkiller containing paracetamol and codeine". National Health Service. 19 December 2018. Archived from the original on 14 January 2022. Retrieved 16 April 2021.
- Nielsen S, Van Hout MC (2017). "Over-the-Counter Codeine-from Therapeutic Use to Dependence, and the Grey Areas in Between". Current Topics in Behavioral Neurosciences. 34: 59–75. doi:10.1007/7854_2015_422. hdl:1959.4/unsworks_62273. ISBN 978-3-319-60014-7. PMID 26768736.
- "Codeine Use While Breastfeeding May Be Dangerous". CTV News. 20 August 2008. Archived from the original on 1 September 2008. Retrieved 13 August 2020.
- Foley M, Harris R, Rich E, Rapca A, Bergin M, Norman I, et al. (November 2015). "The availability of over-the-counter codeine medicines across the European Union". Public Health. 129 (11): 1465–1470. doi:10.1016/j.puhe.2015.06.014. PMID 26215740. Archived from the original on 25 March 2022. Retrieved 8 July 2020.
| Non-steroidal anti-inflammatory drugs (NSAIDs) (primarily M01A and M02A, also N02BA) | |
|---|---|
| pyrazolones / pyrazolidines | |
| salicylates | |
| acetic acid derivatives and related substances | |
| oxicams | |
| propionic acid derivatives (profens) |
|
| n-arylanthranilic acids (fenamates) | |
| COX-2 inhibitors (coxibs) | |
| other | |
| NSAID combinations | |
| Key: underline indicates initially developed first-in-class compound of specific group; WHO-Essential Medicines; withdrawn drugs; veterinary use. | |
| Opioid receptor modulators | |||||
|---|---|---|---|---|---|
| μ-opioid (MOR) |
| ||||
| δ-opioid (DOR) |
| ||||
| κ-opioid (KOR) |
| ||||
| Nociceptin (NOP) |
| ||||
| Others |
| ||||