| Revision as of 15:12, 2 December 2011 editZéroBot (talk | contribs)704,777 editsm r2.7.1) (Robot: Adding uk:Галантамін← Previous edit | Latest revision as of 22:31, 22 November 2024 edit undoJakespeedorg (talk | contribs)8 editsm Linking ketoconazole to its own article on Misplaced Pages | ||
| (321 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Neurological medication}} | |||
| {{Drugbox | |||
| {{Use mdy dates|date=August 2024}} | |||
| | verifiedrevid = 443831212 | |||
| {{cs1 config|name-list-style=vanc|display-authors=6}} | |||
| | IUPAC_name = (4a''S'',6''R'',8a''S'')- 5,6,9,10,11,12- hexahydro- 3-methoxy- 11-methyl- 4a''H''- benzofuro benzazepin- 6-ol | |||
| {{Infobox drug | |||
| | image = Galantamine.svg | |||
| | Watchedfields = changed | |||
| | width = 175 | |||
| | verifiedrevid = 461118063 | |||
| | image = Galantamine.svg | |||
| | alt = | |||
| | image2 = File:Galantamine 3D.png | |||
| | alt2 = | |||
| <!--Clinical data--> | <!--Clinical data--> | ||
| | tradename = Razadyne | | tradename = Razadyne, others | ||
| | Drugs.com = {{drugs.com|monograph| |
| Drugs.com = {{drugs.com|monograph|galantamine-hydrobromide}} | ||
| | MedlinePlus = a699058 | | MedlinePlus = a699058 | ||
| | DailyMedID = Razadyne | |||
| | pregnancy_category = B | |||
| | pregnancy_AU = B1 | |||
| | legal_status = Rx | |||
| | pregnancy_AU_comment = <ref name="Drugs.com pregnancy">{{cite web | title=Galantamine Use During Pregnancy | website=Drugs.com | date=February 18, 2019 | url=https://www.drugs.com/pregnancy/galantamine.html | access-date=February 24, 2020 | archive-date=February 25, 2020 | archive-url=https://web.archive.org/web/20200225040154/https://www.drugs.com/pregnancy/galantamine.html | url-status=live }}</ref> | |||
| | routes_of_administration = Oral | |||
| | pregnancy_category = | |||
| | routes_of_administration = ] | |||
| | ATC_prefix = N06 | |||
| | ATC_suffix = DA04 | |||
| <!-- Legal status --> | |||
| | legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | |||
| | legal_BR = C1 | |||
| | legal_BR_comment = <ref>{{Cite web |author=Anvisa |author-link=Brazilian Health Regulatory Agency |date=March 31, 2023 |title=RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial |trans-title=Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control|url=https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-784-de-31-de-marco-de-2023-474904992 |url-status=live |archive-url=https://web.archive.org/web/20230803143925/https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-784-de-31-de-marco-de-2023-474904992 |archive-date=August 3, 2023 |access-date=August 16, 2023 |publisher=] |language=pt-BR |publication-date=April 4, 2023}}</ref> | |||
| | legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| | legal_DE = <!-- Anlage I, II, III or Unscheduled --> | |||
| | legal_NZ = <!-- Class A, B, C --> | |||
| | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | |||
| | legal_US = Rx-only | |||
| | legal_US_comment = <ref name="Razadyne FDA label">{{cite web | title=Razadyne- galantamine hydrobromide tablet, film coated | website=DailyMed | date=November 11, 2010 | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e62efb5a-d2cc-4e11-9e61-10e65ef3d897 | access-date=August 5, 2024 | archive-date=April 26, 2023 | archive-url=https://web.archive.org/web/20230426034728/https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e62efb5a-d2cc-4e11-9e61-10e65ef3d897 | url-status=live }}</ref> | |||
| | legal_EU = Rx-only | |||
| | legal_EU_comment = <ref>{{cite web | title = Active substance: galantamine | work = List of nationally authorised medicinal products, Human Medicines Evaluation Division | publisher = European Medicines Agency | date = November 12, 2020 | url = https://www.ema.europa.eu/documents/psusa/galantamine-list-nationally-authorised-medicinal-products-psusa/00001512/202003_en.pdf | access-date = December 19, 2020 | archive-date = October 31, 2021 | archive-url = https://web.archive.org/web/20211031194913/https://www.ema.europa.eu/en/documents/psusa/galantamine-list-nationally-authorised-medicinal-products-psusa/00001512/202003_en.pdf | url-status = live }}</ref> | |||
| | legal_status = Rx-only | |||
| <!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| | bioavailability |
| bioavailability = 80–100% | ||
| | protein_bound = 18% | | protein_bound = 18% | ||
| | metabolism = ] partially ]:]/] substrate | | metabolism = ] partially ]:]/] substrate | ||
| | elimination_half-life = 7 hours | | elimination_half-life = 7 hours | ||
| | excretion = ] (95%, of which 32% unchanged), fecal (5%) | | excretion = ] (95%, of which 32% unchanged), fecal (5%) | ||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | IUPHAR_ligand = 6693 | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | CAS_number_Ref = {{cascite|correct|??}} | | CAS_number_Ref = {{cascite|correct|??}} | ||
| | CAS_number = 357-70-0 | | CAS_number = 357-70-0 | ||
| | PubChem = 9651 | |||
| | ATC_prefix = N06 | |||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | ATC_suffix = DA04 | |||
| | DrugBank = DB00674 | |||
| | PubChem = 9651 | |||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 9272 | |||
| | DrugBank = APRD00206 | |||
| | |
| UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = 0D3Q044KCA | |||
| | ChemSpiderID = 9272 | |||
| | |
| KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = D04292 | |||
| | UNII = 0D3Q044KCA | |||
| | |
| ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | ChEBI = 42944 | |||
| | KEGG = D04292 | |||
| | |
| ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | ChEMBL = 659 | |||
| | ChEBI = 42944 | |||
| | PDB_ligand = GNT | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | ChEMBL = 659 | |||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | IUPAC_name = (4a''S'',6''R'',8a''S'')-5,6,9,10,11,12-Hexahydro-3-methoxy-11-methyl-4a''H''-benzofuro benzazepin-6-ol | |||
| | C=17 | H=21 | N=1 | O=3 | |||
| | C = 17 | |||
| | molecular_weight = 287.354 g/mol | |||
| | H = 21 | |||
| | smiles = O(c2c1O4C(O)/C=C\43c1c(cc2)CN(C)CC3)C | |||
| | N = 1 | |||
| | InChI = 1/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | |||
| | O = 3 | |||
| | InChIKey = ASUTZQLVASHGKV-JDFRZJQEBY | |||
| | smiles = O(c2c1O4C(O)/C=C\43c1c(cc2)CN(C)CC3)C | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI = 1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | |||
| | StdInChI = 1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey = ASUTZQLVASHGKV-JDFRZJQESA-N | |||
| | StdInChIKey = ASUTZQLVASHGKV-JDFRZJQESA-N | |||
| | melting_point = 126.5 | |||
| | melting_point = 126.5 | |||
| }} | }} | ||
| '''Galantamine''' |
'''Galantamine''' is a type of ]. It is an ] ]ed from the bulbs and flowers of '']'' (common snowdrop), ''Galanthus caucasicus'' (Caucasian ]), '']'' (Voronov's snowdrop), and other members of the family '']'', such as ''Narcissus'' (]), '']'' (snowflake), and ''Lycoris'' including '']'' (red spider lily).<ref>{{cite web | vauthors = Theodorou M | url = http://www.nnfcc.co.uk/publications/nnfcc-project-factsheet-sustainable-production-of-the-natural-product-galanthamine-defra-nf0612 | archive-url = https://web.archive.org/web/20120314175551/http://www.nnfcc.co.uk/publications/nnfcc-project-factsheet-sustainable-production-of-the-natural-product-galanthamine-defra-nf0612 | archive-date = March 14, 2012 | work = NNFCC Project Factsheet | publisher = The National Non-Food Crops Centre (NNFCC) | title = Sustainable Production of the Natural Product Galanthamine (Defra), NF0612 }}</ref> It can also be ]. | ||
| Galantamine is primarily known for its potential to slow ]. It is used clinically for treating early-stage ] and ] impairments, although it has had limited success with the more advanced condition of ].<ref name="drugs">{{cite web|title=Galantamine|url=https://www.drugs.com/mtm/galantamine.html|publisher=Drugs.com|date=August 8, 2023|access-date=March 26, 2024|archive-date=October 14, 2018|archive-url=https://web.archive.org/web/20181014091614/https://www.drugs.com/mtm/galantamine.html|url-status=live}}</ref><ref name="birks">{{cite journal | vauthors = Birks J | title = Cholinesterase inhibitors for Alzheimer's disease | journal = The Cochrane Database of Systematic Reviews | issue = 1 | pages = CD005593 | date = January 2006 | volume = 2016 | pmid = 16437532 | doi = 10.1002/14651858.CD005593 | pmc = 9006343 | veditors = Birks JS }}</ref><ref name=kalola/><ref name="battle">{{cite journal |vauthors=Battle CE, Abdul-Rahim AH, Shenkin SD, Hewitt J, Quinn TJ |title=Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis |journal=The Cochrane Database of Systematic Reviews |volume=2021 |issue=2 |pages=CD013306 |date=February 2021 |pmid=33704781 |pmc=8407366 |doi=10.1002/14651858.CD013306.pub2}}</ref> | |||
| Studies of usage in modern ] began in the ] in the 1950s. The active ingredient was extracted, identified, and studied, in particular in relation to its ] (AChE)-inhibiting properties. The bulk of the work was carried out by ] pharmacologists Mashkovsky and Kruglikova-Lvova, beginning in 1951.<ref></ref> The work of Mashkovsky and Kruglikova-Lvova was the first published work that demonstrated the AChE-inhibiting properties of galantamine.<ref>Mashkovsky MD, Kruglikova-Lvova RP. On the pharmacology of the new alkaloid galantamine. Farmakologia Toxicologia (Moscow) 1951;14:27-30 (in Russian).</ref> | |||
| It works by increasing the amount of a type of ] named ] by the inhibiting activity of an enzyme called ] known for ] acetylcholine. This elevates and prolongs acetylcholine levels boosting acetylcholine's ] functionality, subsequently enhancing functionality of the various ] that acetylcholine is involved in, such as memory processing, ], and ].<ref name=drugs/> Galantamine may cause serious ]s, such as stomach bleeding, liver injury or chest pain.<ref name=drugs/><ref name="kalola">{{cite web|vauthors=Kalola UK, Nguyen H|title=Galantamine|date=March 12, 2023|publisher=StatPearls Publishing, US National Library of Medicine|pmid=34662060 |url=https://www.ncbi.nlm.nih.gov/books/NBK574546/|accessdate=March 26, 2024|archive-date=December 26, 2023|archive-url=https://web.archive.org/web/20231226163656/https://www.ncbi.nlm.nih.gov/books/NBK574546/|url-status=live}}</ref> | |||
| The first industrial process was developed in ] by ] in 1959 (], ]) from a species traditionally used as a popular medicine in ], and, thus ,the idea for developing a medicine from these species seems to be based on the local use (i.e., an ]-driven drug discovery).<ref>{{cite journal | last1 = Heinrich | first1 = M. | last2 = Teoh | first2 = H.L. | year = 2004 | title = Galanthamine from snowdrop – the development of a modern drug against Alzheimer's disease from local Caucasian knowledge | url = | journal = Journal of Ethnopharmacology | pmid = 15137996 | volume = 92 | issue = 2–3| pages = 147–162 | doi = 10.1016/j.jep.2004.02.012 }}</ref><ref>{{cite journal | last1 = Scott | first1 = LJ | last2 = Goa | first2 = KL | title = Galantamine: a review of its use in Alzheimer's disease | journal = Drugs | volume = 60 | issue = 5 | pages = 1095–122 | year = 2000 | pmid = 11129124 }}</ref> | |||
| Galantamine was isolated for the first time from bulbs of '']'' (common snowdrop) in the Soviet Union in the 1940s.<ref>Proskurnina NF, Areshknina LY. J. Chim. Gen. USSR. Chem. Abst. 1947;1948;1742(1595h):1216. No title available.</ref> The active ingredient was extracted, identified, and studied, in particular in relation to ] (AChE)-inhibiting properties.<ref>{{cite journal | title=Snowdrops: The heralds of spring and a modern drug for Alzheimer's disease | vauthors=Heinrich M | journal=Pharmaceutical Journal | year=2004 | volume=273 | issue=7330 | pages=905–6 | oclc=98892008 | url=http://www.pharmaceutical-journal.com/pj-online-christmas-2004-snowdrops-the-heralds-of-spring-and-a-modern-drug-for-alzheimer8217s-disease/20013614.article | access-date=July 30, 2015 | archive-date=October 23, 2018 | archive-url=https://web.archive.org/web/20181023160656/https://www.pharmaceutical-journal.com/pj-online-christmas-2004-snowdrops-the-heralds-of-spring-and-a-modern-drug-for-alzheimer8217s-disease/20013614.article | url-status=dead }}</ref><ref>{{cite journal | title=On the pharmacology of the new alkaloid galantamine | vauthors = Mashkovsky MD, Kruglikova-Lvova RP | journal=Farmakologia Toxicologia | year=1951 | volume=14 | pages=27–30 }}</ref> The first industrial process was developed in 1959.<ref>{{cite journal | vauthors = Heinrich M, Lee Teoh H | title = Galanthamine from snowdrop--the development of a modern drug against Alzheimer's disease from local Caucasian knowledge | journal = Journal of Ethnopharmacology | volume = 92 | issue = 2–3 | pages = 147–162 | date = June 2004 | pmid = 15137996 | doi = 10.1016/j.jep.2004.02.012 }}</ref><ref>{{cite journal | vauthors = Scott LJ, Goa KL | title = Galantamine: a review of its use in Alzheimer's disease | journal = Drugs | volume = 60 | issue = 5 | pages = 1095–1122 | date = November 2000 | pmid = 11129124 | doi = 10.2165/00003495-200060050-00008 | s2cid = 250305879 }}</ref> However, it was not until the 1990s when full-scale synthesis was upscaled and optimized.<ref>{{Cite web|url=https://www.alzforum.org/therapeutics/galantamine|title=Galantamine|website=alzforum|access-date=November 17, 2019|archive-date=November 17, 2019|archive-url=https://web.archive.org/web/20191117153220/https://www.alzforum.org/therapeutics/galantamine|url-status=live}}</ref> | |||
| Galantamine has been used for decades in Eastern Europe and the ] for various indications such as treatment of myasthenia, myopathy, and sensory and motor dysfunction associated with disorders of the central nervous system. Its uses have included symptomatic treatment of ] (]), and it was later deployed by ] as an anti-Alzheimer's medication. | |||
| In the US, it has been sold as a dietary supplement for memory and dream support. | |||
| == |
== Medical uses == | ||
| Galantamine, sold under the brand name '''Razadyne''' among others, is ] for the treatment of mild to moderate ] and Alzheimer's disease.<ref name=drugs/><ref name=birks/> The first person to extract galantamine and theorize its usefulness in medicine, was the Bulgarian chemist ] in 1959. In the United States, it is approved by the ] (FDA) for the treatment of mild to moderate dementia.<ref name=kalola/><ref>{{cite web|title=Galantamine hydrobromide (trademark)|url=https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/021615lbl.pdf|publisher=US Food and Drug Administration|access-date=December 17, 2017|date=2004|archive-date=July 13, 2020|archive-url=https://web.archive.org/web/20200713225426/https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/021615lbl.pdf|url-status=live}}</ref> Galantamine may not be effective for treating mild cognitive impairment.<ref>{{cite journal | vauthors = Tricco AC, Soobiah C, Berliner S, Ho JM, Ng CH, Ashoor HM, Chen MH, Hemmelgarn B, Straus SE | title = Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysis | journal = CMAJ | volume = 185 | issue = 16 | pages = 1393–401 | date = November 2013 | pmid = 24043661 | pmc = 3826344 | doi = 10.1503/cmaj.130451 }}</ref> | |||
| ===Alzheimer's disease=== | |||
| Galantamine in its pure form is a white powder. Galantamine is a ] and ] ]. It reduces the action of AChE and therefore tends to increase the concentration of ] in the brain. It is hypothesized that this action might relieve some of the symptoms of Alzheimer's. It is also an allosteric ligand at nicotinic acetylcholine receptors. | |||
| Alzheimer's disease is characterized by the impairment of ] function.<ref name=drugs/><ref name=kalola/> One hypothesis is that this impairment contributes to the cognitive deficits caused by the disease. This hypothesis forms the basis for use of galantamine as a cholinergic enhancer in the treatment of Alzheimer's.<ref name=drugs/><ref name=kalola/> Galantamine inhibits acetylcholinesterase, an enzyme which ] acetylcholine.<ref name=drugs/><ref name=kalola/> As a result of acetylcholinesterase inhibition, galantamine increases the availability of acetylcholine for synaptic transmission.<ref name=kalola/> Additionally, galantamine binds to the ] sites of ] receptors, which causes a conformational change.<ref name="springer">{{cite journal | vauthors = Farlow MR | title = Clinical pharmacokinetics of galantamine | journal = Clinical Pharmacokinetics | volume = 42 | issue = 15 | pages = 1383–92 | year = 2003 | pmid = 14674789 | doi = 10.2165/00003088-200342150-00005 | s2cid = 36855768 }}</ref> This allosteric modulation increases the nicotinic receptor's response to acetylcholine.<ref name=kalola/> The activation of presynaptic nicotinic receptors increases the release of acetylcholine, further increasing the availability of acetylcholine.<ref name=kalola /> Galantamine's competitive inhibition of acetylcholinesterase and allosteric nicotinic modulation serves as a dual mechanism of action.<ref name="springer" /> | |||
| To reduce the prevalence of negative side effects associated with galantamine, such as ] and ], a dose-escalation scheme may be used.<ref name="direct">{{cite journal | vauthors = Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV | title = Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial | journal = Lancet | volume = 359 | issue = 9314 | pages = 1283–90 | date = April 2002 | pmid = 11965273 | doi = 10.1016/S0140-6736(02)08267-3 | s2cid = 1172847 }}</ref> The use of a dose-escalation scheme has been well accepted in countries where galantamine is used.<ref name="direct" /> A dose-escalation scheme for Alzheimer's treatment involves a recommended starting dosage of 4 mg galantamine tablets given twice a day (8 mg/day).<ref name=drugs/> After a minimum of 4 weeks, the dosage may then be increased to 8 mg given twice a day (16 mg/day).<ref name="drugs" /> After a minimum of 4 weeks at 16 mg/day, the treatment may be increased to 12 mg given twice a day (24 mg/day).<ref name="drugs" /> Dosage increases are based upon the assessment of clinical benefit as well as tolerability of the previous dosage.<ref name="drugs" /> If treatment is interrupted for more than three days, the process is usually restarted, beginning at the starting dosage, and re-escalating to the current dose.<ref name="drugs" /> It has been found that a dosage between 16–24 mg/day is the optimal dosage.<ref name="pmid24591834">{{cite journal | vauthors = Hager K, Baseman AS, Nye JS, Brashear HR, Han J, Sano M, Davis B, Richards HM | title = Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer's disease | journal = Neuropsychiatric Disease and Treatment | volume = 10 | pages = 391–401 | date = 2014 | pmid = 24591834 | pmc = 3937252 | doi = 10.2147/NDT.S57909 | doi-access = free }}</ref> | |||
| The atomic resolution 3D structure of the complex of galantamine and its target, acetylcholinesterase, was determined by ] in 1999 (PDB code: ; ).<ref>{{cite journal | doi = 10.1016/S0014-5793(99)01637-3 | last1 = Greenblatt | first1 = HM | last2 = Kryger | first2 = G | last3 = Lewis | first3 = T | last4 = Silman | first4 = I | last5 = Sussman | first5 = JL | year = 1999 | title = Structure of acetylcholinesterase complexed with (-)-galanthamine at 2.3Å resolution | url = | journal = FEBS Lett | volume = 463 | issue = 3| pages = 321–26 | pmid = 10606746 }}</ref> There is no evidence that galantamine alters the course of the underlying dementing process.<ref></ref> Galantamine has also shown activity in modulating the ]s on cholinergic neurons to increase acetylcholine release.<ref>{{cite journal | last1 = Woodruff-Pak | first1 = DS | last2 = Vogel Rw | first2 = 3rd | last3 = Wenk | first3 = GL | title = Galantamine: Effect on nicotinic receptor binding, acetylcholinesterase inhibition, and learning | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 98 | issue = 4 | pages = 2089–94 | year = 2001 | pmid = 11172080 | pmc = 29386 | doi = 10.1073/pnas.031584398 }}</ref> | |||
| In December 2023, the FDA approved a ] (NDA) for a pro-drug of galantamine called ALPHA-1062.<ref>{{cite press release | title=Alpha Cognition Announces FDA Acceptance of New Drug Application for ALPHA-1062 for Mild-to-Moderate Alzheimer's Disease | publisher=Alpha Cognition | via=Business Wire | date=December 7, 2023 | url=https://www.businesswire.com/news/home/20231207840612/en/Alpha-Cognition-Announces-FDA-Acceptance-of-New-Drug-Application-for-ALPHA-1062-for-Mild-to-Moderate-Alzheimer%E2%80%99s-Disease | access-date=August 5, 2024 | archive-date=April 16, 2024 | archive-url=https://web.archive.org/web/20240416023157/https://www.businesswire.com/news/home/20231207840612/en/Alpha-Cognition-Announces-FDA-Acceptance-of-New-Drug-Application-for-ALPHA-1062-for-Mild-to-Moderate-Alzheimer%E2%80%99s-Disease | url-status=live }}</ref> In July 2024, The FDA approved ] (Zunveyl), previously known as ALPHA-1062, to treat mild-to-moderate Alzheimer's disease.<ref>{{cite press release | title=Alpha Cognition's Oral Therapy Zunveyl Receives FDA Approval to Treat Alzheimer's Disease | publisher=Alpha Cognition | via=Business Wire | date=July 29, 2024 | url=https://www.businesswire.com/news/home/20240729327804/en/ | access-date=August 4, 2024 | archive-date=August 4, 2024 | archive-url=https://web.archive.org/web/20240804193100/https://www.businesswire.com/news/home/20240729327804/en/ | url-status=live }}</ref> | |||
| == Pharmacokinetics == | |||
| == Side effects == | |||
| Absorption of galantamine is rapid and complete and shows linear pharmacokinetics. It is well absorbed with absolute oral ] between 80 and 100%. It has a ] of seven hours. Peak effect of inhibiting ] was achieved about one hour after a single oral dose of 8 mg in some healthy volunteers. | |||
| The ] profile of galantamine includes potential for ], including ], swelling of the face or throat, and skin rash.<ref name=drugs/><ref name=mayo/> Using galantamine may cause chest pain, bloody urine, stomach bleeding, and liver injury, among other side effects.<ref name=drugs/><ref name=mayo/> Nausea, vomiting, diarrhea, dizziness, and headache are considered common side effects.<ref name="drugs" /> | |||
| A gradual titration over more than three months may enable long-term tolerability in some people.<ref>{{cite journal | vauthors = Birks J | title = Cholinesterase inhibitors for Alzheimer's disease | journal = The Cochrane Database of Systematic Reviews | issue = 1 | pages = CD005593 | date = January 2006 | volume = 2016 | pmid = 16437532 | doi = 10.1002/14651858.CD005593 | pmc = 9006343 | veditors = Birks J }}</ref> | |||
| ] of galantamine is about 18%, which is relatively low. | |||
| Galantamine has a wide spectrum of ] and medical disorders, requiring close assessment between the physician and patient.<ref name="mayo">{{cite web |title=Galantamine |url=https://www.mayoclinic.org/drugs-supplements/galantamine-oral-route/precautions/drg-20067458?p=1 |publisher=Mayo Clinic |access-date=March 26, 2024 |date=2024 |archive-date=March 26, 2024 |archive-url=https://web.archive.org/web/20240326145046/https://www.mayoclinic.org/drugs-supplements/galantamine-oral-route/precautions/drg-20067458?p=1 |url-status=live }}</ref> | |||
| == Metabolism == | |||
| == Pharmacology == | |||
| Approximately 75% of a dose of galantamine is metabolised in the liver. In vitro studies have shown that Hepatic ] and ] are involved in galantamine metabolism. | |||
| Galantamine's chemical structure contains a tertiary ]. At a neutral ], this tertiary amine will often bond to a hydrogen, and appear mostly as an ammonium ion.<ref name=drugs/> | |||
| Galantamine is a potent ] potentiating ] of human ] (nAChRs) ], ], and ], and chicken/mouse nAChRs ]/] in certain areas of the brain.<ref name=drugs/><ref name="metabolism">{{cite journal | vauthors = Mannens GS, Snel CA, Hendrickx J, Verhaeghe T, Le Jeune L, Bode W, van Beijsterveldt L, Lavrijsen K, Leempoels J, Van Osselaer N, Van Peer A, Meuldermans W | title = The metabolism and excretion of galantamine in rats, dogs, and humans | journal = Drug Metabolism and Disposition | volume = 30 | issue = 5 | pages = 553–63 | date = May 2002 | pmid = 11950787 | doi = 10.1124/dmd.30.5.553 | s2cid = 6795456 }}</ref> By binding to the allosteric site of the nAChRs, a conformational change occurs which increases the receptors response to acetylcholine.<ref name=kalola/> This modulation of the ]s on cholinergic neurons in turn causes an increase in the amount of acetylcholine released.<ref>{{cite journal | vauthors = Woodruff-Pak DS, Vogel RW, Wenk GL | title = Galantamine: effect on nicotinic receptor binding, acetylcholinesterase inhibition, and learning | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 98 | issue = 4 | pages = 2089–94 | date = February 2001 | pmid = 11172080 | pmc = 29386 | doi = 10.1073/pnas.031584398 | bibcode = 2001PNAS...98.2089W | jstor = 3055005 | doi-access = free }}</ref> | |||
| For Razadyne ER (the once-a-day formulation), CYP2D6 ]s had drug exposures that were approximately 50% higher than for extensive metabolizers. About 7% of the population has this genetic mutation; however, because the drug is individually titrated to tolerability, no specific dosage adjustment is necessary for this population. | |||
| However, recent studies suggest that Galantamine does not functionally act at human nAChRs ] or ] as a positive allosteric modulator.<ref>{{cite journal | vauthors = Moerke MJ, McMahon LR, Wilkerson JL | title = More than Smoke and Patches: The Quest for Pharmacotherapies to Treat Tobacco Use Disorder | journal = Pharmacological Reviews | volume = 72 | issue = 2 | pages = 527–557 | date = April 2020 | pmid = 32205338 | pmc = 7090325 | doi = 10.1124/pr.119.018028 | url = https://pharmrev.aspetjournals.org/content/72/2/527/tab-article-info | access-date = October 24, 2020 | archive-date = October 8, 2021 | archive-url = https://web.archive.org/web/20211008220759/https://pharmrev.aspetjournals.org/content/72/2/527/tab-article-info | url-status = live }}</ref><ref name="pmid29669164">{{cite journal |vauthors=Kowal NM, Ahring PK, Liao WY, Indurti DC, Harvey BS, O'Connor SM, Chebib M, Olafsdottir ES, Balle T |title=Galantamine is not a positive allosteric modulator of human α4β2 or α7 nicotinic acetylcholine receptors |journal=] |volume=175 |issue=14 |pages=2911–2925 |date=July 2018 |pmid=29669164 |pmc=6016680 |doi=10.1111/bph.14329 |url= |issn=}}</ref> | |||
| Galantamine also works as a weak ] and ] ] in all areas of the body.<ref name="drugs" /> By inhibiting acetylcholinesterase, it increases the concentration and thereby action of ] in certain parts of the brain. Galantamine's effects on nAChRs and complementary acetylcholinesterase inhibition make up a dual mechanism of action. It is hypothesized that this action might relieve some of the symptoms of Alzheimer's. | |||
| == Clinical use == | |||
| === Indications === | |||
| ] | |||
| Galantamine is indicated for the treatment of mild to moderate ] and Alzheimer's.<ref></ref><ref></ref> | |||
| Galantamine in its pure form is a white powder. The atomic resolution 3D structure of the complex of galantamine and its target, acetylcholinesterase, was determined by ] in 1999 (PDB code: ; ).<ref>{{cite journal | vauthors = Greenblatt HM, Kryger G, Lewis T, Silman I, Sussman JL | title = Structure of acetylcholinesterase complexed with (-)-galanthamine at 2.3 A resolution | journal = FEBS Letters | volume = 463 | issue = 3 | pages = 321–6 | date = December 1999 | pmid = 10606746 | doi = 10.1016/S0014-5793(99)01637-3 | s2cid = 573270 | doi-access = free | bibcode = 1999FEBSL.463..321G }}</ref> There is no evidence that galantamine alters the course of the underlying dementing process.<ref>{{Cite web |url=http://www.ortho-mcneilneurologics.com/ortho-mcneilneurologics/shared/pi/razadyne.pdf |title=Ortho-McNeil Neurologics, "Razadyne ER US Product Insert", May 2006 |access-date=December 21, 2009 |archive-url=https://web.archive.org/web/20091222071643/http://www.ortho-mcneilneurologics.com/ortho-mcneilneurologics/shared/pi/razadyne.pdf |archive-date=December 22, 2009 |url-status=dead }}</ref> | |||
| === Available forms === | |||
| == Pharmacokinetics == | |||
| The product is supplied in twice-a-day tablets, in once-a-day extended-release capsules, and in oral solution. The tablets come in 4 mg, 8 mg, and 12 mg forms. The capsules come in 8 mg, 16 mg, and 24 mg forms. | |||
| Absorption of galantamine is rapid and complete and shows linear pharmacokinetics. It is well absorbed with absolute oral ] between 80 and 100%. It has a terminal elimination half-life of seven hours. Peak effect of inhibiting ] was achieved about one hour after a single oral dose of 8 mg in some healthy volunteers. | |||
| === Adverse events === | |||
| The coadministration of food delays the rate of galantamine absorption, but does not affect the extent of absorption.<ref name="springer" /> | |||
| In clinical trials, galantamine's ] profile was very similar to that of other cholinesterase inhibitors, with ] symptoms being the most notable and most commonly observed. In practice, some other cholinesterase inhibitors might be better tolerated; however, a careful and gradual titration over more than three months may lead to equivalent long-term tolerability.<ref>{{cite journal | last1 = Birks | first1 = J | last2 = Birks | first2 = Jacqueline | title = Cholinesterase inhibitors for Alzheimer's disease | journal = Cochrane database of systematic reviews (Online) | issue = 1 | pages = CD005593 | year = 2006 | pmid = 16437532 | doi = 10.1002/14651858.CD005593 | editor1-last = Birks | editor1-first = Jacqueline }}</ref> | |||
| ] of galantamine is about 18%, which is relatively low. | |||
| == Other uses == | |||
| === Supplement for lucid dream and out-of-body experience === | |||
| == Metabolism == | |||
| Some people who practice ] (LD) or ] (OBE) use galantamine to increase their odds to achieve LD or OBE.<ref name=Yuschak2006>{{cite book | author = Thomas Yuschak | title = Advanced Lucid Dreaming | edition = 1st | publisher = Lulu Enterprises | year = 2006 |isbn = 978-1-4303-0542-2 }}</ref><ref name=Yuschak2007>{{cite book | author = Thomas Yuschak | title = Pharmacological Induction of Lucid dreams | year = 2007 | url = http://www.advancedld.com/f/Pharmacological_Induction_of_Lucid_Dreams.pdf}}</ref><ref name=LaBerge2004>{{cite web | title= Substances that enhance recall and lucidity during dreaming | work=Stephen LaBerge - US Patent | url=http://www.freepatentsonline.com/20040266659.html | accessdate=2007-10-29}}</ref> | |||
| By taking small amount of galantamine (around 4 to 8 mg) after five to six hours of deep sleep and practice an induction technique such as ], MILD or WILD many people report more success with galantamine.<ref name=YuschakSite>{{cite web | title=Galantamine LDS Profile | work=Yuschak LDS Profiles | url=http://www.advancedld.com/galantamine.html | accessdate=2007-10-29}}</ref> | |||
| Approximately 75% of a dose of galantamine is metabolised in the liver. In vitro studies have shown that hepatic ] and ] are involved in galantamine metabolism. Within 24 hours of intravenous or oral administration approximately 20% of a dose of galantamine will be excreted unreacted in the urine.<ref name="springer" /> | |||
| There are also reports claiming that taking galantamine without proper induction technique will not lead to LD or OBE but will result in only a vivid dream instead. | |||
| It should also be noted that, due to a long half-life, galantamine will stay in the body for a period of up to and over 48 hours. As such, it is advisable to space out the use of galantamine over a period of three days so that the body does not build a resistance to the drug, ruining its effectiveness.<ref name="Yuschak2006"/> | |||
| In humans, several metabolic pathways for galantamine exist.<ref name=metabolism/> These pathways lead to the formation of a number of different metabolites.<ref name=metabolism/> One of the metabolites that may result can be formed through the ] of galantamine.<ref name=metabolism/> Additionally, galantamine may undergo ] or ] at its nitrogen atom, forming two other possible metabolites.<ref name=metabolism/> Galantamine can undergo demethylation at its oxygen atom, forming an intermediate which can then undergo glucuronidation or sulfate conjugation.<ref name=metabolism/> Lastly, galantamine may be oxidized and then reduced before finally undergoing demethylation or oxidation at its nitrogen atom, or demethylation and subsequent glucuronidation at its oxygen atom.<ref name=metabolism/> | |||
| Galantamine used with ] or ] can dramatically increase one's odds of becoming lucid and increase memory consolidation during dreaming.{{Citation needed|date=July 2008}} | |||
| Some people report mixing galantamine with other ] can enhance the degree of lucidity, but this is still controversial, since some mixtures may work for some people, but lead to failure for others. | |||
| ] | |||
| === Sleep aid === | |||
| ==Drug interactions== | |||
| Galantamine has been used as a sleep aid, Galantamine has been anecdotally described both to help people fall asleep and increasing the quality of sleep once someone falls asleep. Extensive research and sleep studies have not been conducted, but initial microbiological research suggests that Galantamine does have properties that can aid patients suffering from insomnia. | |||
| Since galantamine is metabolized by CYP2D6 and CYP3A4, inhibiting either of these ] will increase the cholinergic effects of galantamine.<ref name="springer" /> Inhibiting these enzymes may lead to adverse effects.<ref name="springer" /> It was found that ], an inhibitor of CYP2D6, increased the ] of galantamine by 40%.<ref name="springer" /> The CYP3A4 inhibitors ] and ] increased the ] of galantamine by 30% and 12%, respectively.<ref name="springer" /> | |||
| == Extraction and synthesis == | |||
| === Nootropic === | |||
| {{main|Galantamine total synthesis}} | |||
| Since the alkaloid is isolated from botanical sources containing low amounts (0.1%) by weight, extraction yields are low.<ref name="pmid28337117">{{cite journal | vauthors = Kim JK, Park SU | title = Pharmacological aspects of galantamine for the treatment of Alzheimer's disease | journal = EXCLI Journal | volume = 16 | pages = 35–39 | date = 2017 | pmid = 28337117 | pmc = 5318685 | doi = 10.17179/excli2016-820 }}</ref> Although galantamine can be produced from natural resources, it also has many industrial syntheses, such as by ], ], ], and ].<ref>{{cite journal | vauthors = Mucke HA | title = The case of galantamine: repurposing and late blooming of a cholinergic drug | journal = Future Science OA | volume = 1 | issue = 4 | pages = FSO73 | date = November 2015 | pmid = 28031923 | pmc = 5137937 | doi = 10.4155/fso.15.73 }}</ref> | |||
| ==Research== | |||
| Along with other cholinergics or acetylcholinesterase inhibitors such as ], galantamine also has been used as ] or "brain enhancer" to enhance memory in brain-damaged adults.<ref></ref> | |||
| ===Organophosphate poisoning=== | |||
| == Caution == | |||
| The toxicity of ]s results primarily from their action as irreversible inhibitors of acetylcholinesterase.<ref name = "organo" /> Inhibiting acetylcholinesterase causes an increase in acetylcholine, as the enzyme is no longer available to catalyze its breakdown.<ref name = "organo" /> In the peripheral nervous system, acetylcholine accumulation can cause an overstimulation of muscarinic receptors followed by a desensitization of nicotinic receptors.<ref name = "organo" /> This leads to severe skeletal muscle fasciculations (involuntary contractions).<ref name = "organo" /> The effects on the central nervous system include ], restlessness, ], ], ]s, ], cardiorespiratory paralysis, and ].<ref name = "organo" /> As a reversible acetylcholinesterase inhibitor, galantamine has the potential to serve as an effective organophosphate poisoning treatment by preventing irreversible acetylcholinesterase inhibition.<ref name = "organo" /> Additionally, galantamine has ] properties which may make it even more desirable as an antidote.<ref name = "organo" /> | |||
| Research supported in part by the ] has led to a ] application for the use of galantamine and/or its derivatives for treatment of ].<ref name = "organo">{{cite journal | vauthors = Albuquerque EX, Pereira EF, Aracava Y, Fawcett WP, Oliveira M, Randall WR, Hamilton TA, Kan RK, Romano JA, Adler M | title = Effective countermeasure against poisoning by organophosphorus insecticides and nerve agents | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 103 | issue = 35 | pages = 13220–5 | date = August 2006 | pmid = 16914529 | pmc = 1550772 | doi = 10.1073/pnas.0605370103 | bibcode = 2006PNAS..10313220A | doi-access = free }}</ref> The indications for use of galantamine in the patent application include poisoning by ]s "including but not limited to ], ], and ], ], and ]s". Galantamine was studied in the research cited in the patent application for use along with the well-recognized nerve agent antidote ]. According to the investigators, an unexpected synergistic interaction occurred between galantamine and atropine in an amount of 6 mg/kg or higher. Increasing the dose of galantamine from 5 to 8 mg/kg decreased the dose of atropine needed to protect experimental animals from the toxicity of soman in dosages 1.5 times the dose generally required to kill half the experimental animals.<ref name="Albuquerque_patent">{{cite web |url=http://appft.uspto.gov/netacgi/nph-Parser?p=1&u=%2Fnetahtml%2FPTO%2Fsearch-adv.html&r=1&f=G&l=50&d=PG01&s1=20090023706.PN.&OS=PN/20090023706&RS=PN/20090023706 |title=United States Patent Application 20090023706 |publisher=US Patent and Trademark Office |date=January 22, 2009 |access-date=May 27, 2016 |vauthors=((Albuquerque, Edson X)), ((Adler, Michael)), ((Pereira, Edna F.R.)) |archive-date=July 13, 2020 |archive-url=https://web.archive.org/web/20200713225511/http://appft.uspto.gov/netacgi/nph-Parser?p=1&u=%2Fnetahtml%2FPTO%2Fsearch-adv.html&r=1&f=G&l=50&d=PG01&s1=20090023706.PN.&OS=PN%2F20090023706&RS=PN%2F20090023706 |url-status=dead }}</ref> | |||
| The ] (FDA) and international health authorities have published an alert based on data from two studies during the treatment by galantamine of ]; higher mortality rates were seen in drug-treated patients.<ref>{{cite web|url=http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126138.pdf|title=FDA ALERT: Galantamine hydrobromide (marketed as Razadyne, formerly Reminyl) - Healthcare Professional Sheet|date=May 2005|work=Postmarket Drug Safety Information for Patients and Providers|publisher=]|accessdate=2010-04-02}}</ref><ref>{{cite web|url=http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm152595.htm|title=Safety Alerts for Human Medical Products > Reminyl (galantamine hydrobromide)|date=March 2005|work=# MedWatch The FDA Safety Information and Adverse Event Reporting Program|publisher=]|accessdate=2010-08-04}}</ref> On April 27, 2006, FDA approved labeling changes concerning all form of galantamine preparations (liquid, regular tablets,and extended release tablets) warning of the risk of ] (and sometimes atrioventricular block, especially in predisposed persons). At the same time, the risk of ] seems to be increased relative to placebo.<ref>{{cite web|url=http://www.fda.gov/medwatch/safety/2006/apr06.htm |title=Safety Labeling Changes Approved By FDA Center for Drug Evaluation and Research (CDER) |accessdate=2009-07-30 |date=April 2006 |work=MedWatch, The FDA Safety Information and Adverse Event Reporting Program |publisher=] |archiveurl=http://web.archive.org/web/20071009145003/http://www.fda.gov/medwatch/safety/2006/apr06.htm |archivedate=2007-10-09 }}</ref> These side effects have not been reported in any other studies except {{Clarify|date=July 2011}} in mild cognitive impairment. | |||
| ===Autism=== | |||
| == Total synthesis == | |||
| Galantamine given in addition to ] to autistic children has been shown to improve some of the symptoms of autism such as irritability, lethargy, and social withdrawal.<ref>{{cite journal | vauthors = Ghaleiha A, Ghyasvand M, Mohammadi MR, Farokhnia M, Yadegari N, Tabrizi M, Hajiaghaee R, Yekehtaz H, Akhondzadeh S | title = Galantamine efficacy and tolerability as an augmentative therapy in autistic children: A randomized, double-blind, placebo-controlled trial | journal = Journal of Psychopharmacology | volume = 28 | issue = 7 | pages = 677–85 | date = July 2014 | pmid = 24132248 | doi = 10.1177/0269881113508830 | s2cid = 206491732 }}</ref> Additionally, the cholinergic and nicotinic receptors are believed to play a role in attentional processes.<ref name="aut">{{cite journal | vauthors = Nicolson R, Craven-Thuss B, Smith J | title = A prospective, open-label trial of galantamine in autistic disorder | journal = Journal of Child and Adolescent Psychopharmacology | volume = 16 | issue = 5 | pages = 621–9 | date = October 2006 | pmid = 17069550 | doi = 10.1089/cap.2006.16.621 }}</ref> Some studies have noted that cholinergic and nicotinic treatments have improved attention in autistic children.<ref name="aut" /> As such, it is hypothesized that galantamine's dual action mechanism might have a similar effect in treating autistic children and adolescents.<ref name="aut" /> | |||
| ===Anesthesia=== | |||
| {{main|Galanthamine total synthesis}} | |||
| Galantamine may have some limited use in reducing the side-effects of anesthetics ] and ]. In one study, a control group of patients were given ] and ] and underwent ] and surgery.<ref name="anesth">{{cite journal | vauthors = Chakalova E, Marinova M, Srebreva M, Anastasov D, Ploskov K | title = | language = bg | journal = Akusherstvo I Ginekologiia | volume = 26 | issue = 3 | pages = 28–31 | date = 1987 | pmid = 3631427 }}</ref> The experimental group was given ], ], and nivalin (of which the active ingredient is galantamine).<ref name="anesth" /> The degree of drowsiness and disorientation of the two groups was then assessed 5, 10, 15, 30 and 60 minutes after surgery.<ref name="anesth" /> The group that had taken nivalin were found to be more alert 5, 10, and 15 minutes after the surgery.<ref name="anesth" /> | |||
| === Oneirogen === | |||
| Galantamine is produced from natural resources and a patented ] process. Many other synthetic methods exist but have not been implemented on an industrial scale. | |||
| Galantamine is known to have ] properties. Research has demonstrated its potential to increase ], dream self-awareness and dream vividness. The enhancement of such ] properties can facilitate the induction of ].<ref name="Tan Fan 2023 p. ">{{cite journal | vauthors = Tan S, Fan J | title = A systematic review of new empirical data on lucid dream induction techniques | journal = Journal of Sleep Research | volume = 32 | issue = 3 | pages = e13786 | date = June 2023 | pmid = 36408823 | doi = 10.1111/jsr.13786 }}</ref><ref name="Sparrow Hurd Carlson Molina 2018 pp. 74–88">{{cite journal | vauthors = Sparrow G, Hurd R, Carlson R, Molina A | title = Exploring the effects of galantamine paired with meditation and dream reliving on recalled dreams: Toward an integrated protocol for lucid dream induction and nightmare resolution | journal = Consciousness and Cognition | volume = 63 | pages = 74–88 | date = August 2018 | pmid = 29960246 | doi = 10.1016/j.concog.2018.05.012 | publisher = Elsevier BV }}</ref> | |||
| == References == | |||
| {{Reflist|2}} | |||
| == |
== References == | ||
| {{Reflist}} | |||
| * (galantamine compound website) | |||
| * (manufacturer's website) | |||
| * (patient information) | |||
| *{{Proteopedia|1dx6}} | |||
| * | |||
| {{Antidementia}} | {{Antidementia}} | ||
| {{Acetylcholine metabolism and transport modulators}} | |||
| {{Nootropics}} | |||
| {{Nicotinic acetylcholine receptor modulators}} | |||
| {{Cholinergics}} | |||
| {{Portal bar | Medicine}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 22:31, 22 November 2024
Neurological medicationPharmaceutical compound
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Razadyne, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699058 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80–100% |
| Protein binding | 18% |
| Metabolism | Liver partially CYP450:CYP2D6/3A4 substrate |
| Elimination half-life | 7 hours |
| Excretion | Kidney (95%, of which 32% unchanged), fecal (5%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.118.289 |
| Chemical and physical data | |
| Formula | C17H21NO3 |
| Molar mass | 287.359 g·mol |
| 3D model (JSmol) | |
| Melting point | 126.5 °C (259.7 °F) |
SMILES
| |
InChI
| |
| (verify) | |
Galantamine is a type of acetylcholinesterase inhibitor. It is an alkaloid extracted from the bulbs and flowers of Galanthus nivalis (common snowdrop), Galanthus caucasicus (Caucasian snowdrop), Galanthus woronowii (Voronov's snowdrop), and other members of the family Amaryllidaceae, such as Narcissus (daffodil), Leucojum aestivum (snowflake), and Lycoris including Lycoris radiata (red spider lily). It can also be produced synthetically.
Galantamine is primarily known for its potential to slow cognitive decline. It is used clinically for treating early-stage Alzheimer's disease and memory impairments, although it has had limited success with the more advanced condition of dementia.
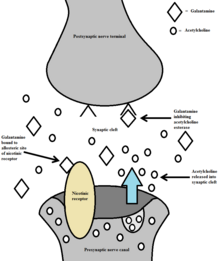
It works by increasing the amount of a type of neurotransmitter named acetylcholine by the inhibiting activity of an enzyme called acetylcholinesterase known for breaking down acetylcholine. This elevates and prolongs acetylcholine levels boosting acetylcholine's neuromodulatory functionality, subsequently enhancing functionality of the various cognitions that acetylcholine is involved in, such as memory processing, reasoning, and thinking. Galantamine may cause serious adverse effects, such as stomach bleeding, liver injury or chest pain.
Galantamine was isolated for the first time from bulbs of Galanthus nivalis (common snowdrop) in the Soviet Union in the 1940s. The active ingredient was extracted, identified, and studied, in particular in relation to acetylcholinesterase (AChE)-inhibiting properties. The first industrial process was developed in 1959. However, it was not until the 1990s when full-scale synthesis was upscaled and optimized.
Medical uses
Galantamine, sold under the brand name Razadyne among others, is indicated for the treatment of mild to moderate vascular dementia and Alzheimer's disease. The first person to extract galantamine and theorize its usefulness in medicine, was the Bulgarian chemist Dimitar Paskov in 1959. In the United States, it is approved by the Food and Drug Administration (FDA) for the treatment of mild to moderate dementia. Galantamine may not be effective for treating mild cognitive impairment.
Alzheimer's disease
Alzheimer's disease is characterized by the impairment of cholinergic function. One hypothesis is that this impairment contributes to the cognitive deficits caused by the disease. This hypothesis forms the basis for use of galantamine as a cholinergic enhancer in the treatment of Alzheimer's. Galantamine inhibits acetylcholinesterase, an enzyme which hydrolyzes acetylcholine. As a result of acetylcholinesterase inhibition, galantamine increases the availability of acetylcholine for synaptic transmission. Additionally, galantamine binds to the allosteric sites of nicotinic receptors, which causes a conformational change. This allosteric modulation increases the nicotinic receptor's response to acetylcholine. The activation of presynaptic nicotinic receptors increases the release of acetylcholine, further increasing the availability of acetylcholine. Galantamine's competitive inhibition of acetylcholinesterase and allosteric nicotinic modulation serves as a dual mechanism of action.
To reduce the prevalence of negative side effects associated with galantamine, such as nausea and vomiting, a dose-escalation scheme may be used. The use of a dose-escalation scheme has been well accepted in countries where galantamine is used. A dose-escalation scheme for Alzheimer's treatment involves a recommended starting dosage of 4 mg galantamine tablets given twice a day (8 mg/day). After a minimum of 4 weeks, the dosage may then be increased to 8 mg given twice a day (16 mg/day). After a minimum of 4 weeks at 16 mg/day, the treatment may be increased to 12 mg given twice a day (24 mg/day). Dosage increases are based upon the assessment of clinical benefit as well as tolerability of the previous dosage. If treatment is interrupted for more than three days, the process is usually restarted, beginning at the starting dosage, and re-escalating to the current dose. It has been found that a dosage between 16–24 mg/day is the optimal dosage.
In December 2023, the FDA approved a New Drug Application (NDA) for a pro-drug of galantamine called ALPHA-1062. In July 2024, The FDA approved benzgalantamine (Zunveyl), previously known as ALPHA-1062, to treat mild-to-moderate Alzheimer's disease.
Side effects
The adverse effect profile of galantamine includes potential for allergic reaction, including hives, swelling of the face or throat, and skin rash. Using galantamine may cause chest pain, bloody urine, stomach bleeding, and liver injury, among other side effects. Nausea, vomiting, diarrhea, dizziness, and headache are considered common side effects.
A gradual titration over more than three months may enable long-term tolerability in some people.
Galantamine has a wide spectrum of interactions with other medications and medical disorders, requiring close assessment between the physician and patient.
Pharmacology
Galantamine's chemical structure contains a tertiary amine. At a neutral pH, this tertiary amine will often bond to a hydrogen, and appear mostly as an ammonium ion.
Galantamine is a potent allosteric potentiating ligand of human nicotinic acetylcholine receptors (nAChRs) α4β2, α3β4, and α6β4, and chicken/mouse nAChRs α7/5-HT3 in certain areas of the brain. By binding to the allosteric site of the nAChRs, a conformational change occurs which increases the receptors response to acetylcholine. This modulation of the nicotinic cholinergic receptors on cholinergic neurons in turn causes an increase in the amount of acetylcholine released. However, recent studies suggest that Galantamine does not functionally act at human nAChRs α4β2 or α7 as a positive allosteric modulator.
Galantamine also works as a weak competitive and reversible cholinesterase inhibitor in all areas of the body. By inhibiting acetylcholinesterase, it increases the concentration and thereby action of acetylcholine in certain parts of the brain. Galantamine's effects on nAChRs and complementary acetylcholinesterase inhibition make up a dual mechanism of action. It is hypothesized that this action might relieve some of the symptoms of Alzheimer's.
Galantamine in its pure form is a white powder. The atomic resolution 3D structure of the complex of galantamine and its target, acetylcholinesterase, was determined by X-ray crystallography in 1999 (PDB code: 1DX6; see complex). There is no evidence that galantamine alters the course of the underlying dementing process.
Pharmacokinetics
Absorption of galantamine is rapid and complete and shows linear pharmacokinetics. It is well absorbed with absolute oral bioavailability between 80 and 100%. It has a terminal elimination half-life of seven hours. Peak effect of inhibiting acetylcholinesterase was achieved about one hour after a single oral dose of 8 mg in some healthy volunteers.
The coadministration of food delays the rate of galantamine absorption, but does not affect the extent of absorption.
Plasma protein binding of galantamine is about 18%, which is relatively low.
Metabolism
Approximately 75% of a dose of galantamine is metabolised in the liver. In vitro studies have shown that hepatic CYP2D6 and CYP3A4 are involved in galantamine metabolism. Within 24 hours of intravenous or oral administration approximately 20% of a dose of galantamine will be excreted unreacted in the urine.
In humans, several metabolic pathways for galantamine exist. These pathways lead to the formation of a number of different metabolites. One of the metabolites that may result can be formed through the glucuronidation of galantamine. Additionally, galantamine may undergo oxidation or demethylation at its nitrogen atom, forming two other possible metabolites. Galantamine can undergo demethylation at its oxygen atom, forming an intermediate which can then undergo glucuronidation or sulfate conjugation. Lastly, galantamine may be oxidized and then reduced before finally undergoing demethylation or oxidation at its nitrogen atom, or demethylation and subsequent glucuronidation at its oxygen atom.

Drug interactions
Since galantamine is metabolized by CYP2D6 and CYP3A4, inhibiting either of these isoenzymes will increase the cholinergic effects of galantamine. Inhibiting these enzymes may lead to adverse effects. It was found that paroxetine, an inhibitor of CYP2D6, increased the bioavailability of galantamine by 40%. The CYP3A4 inhibitors ketoconazole and erythromycin increased the bioavailability of galantamine by 30% and 12%, respectively.
Extraction and synthesis
Main article: Galantamine total synthesisSince the alkaloid is isolated from botanical sources containing low amounts (0.1%) by weight, extraction yields are low. Although galantamine can be produced from natural resources, it also has many industrial syntheses, such as by Janssen, Ortho-McNeil Pharmaceutical, Shire, and Takeda Pharmaceutical Company.
Research
Organophosphate poisoning
The toxicity of organophosphates results primarily from their action as irreversible inhibitors of acetylcholinesterase. Inhibiting acetylcholinesterase causes an increase in acetylcholine, as the enzyme is no longer available to catalyze its breakdown. In the peripheral nervous system, acetylcholine accumulation can cause an overstimulation of muscarinic receptors followed by a desensitization of nicotinic receptors. This leads to severe skeletal muscle fasciculations (involuntary contractions). The effects on the central nervous system include anxiety, restlessness, confusion, ataxia, tremors, seizures, cardiorespiratory paralysis, and coma. As a reversible acetylcholinesterase inhibitor, galantamine has the potential to serve as an effective organophosphate poisoning treatment by preventing irreversible acetylcholinesterase inhibition. Additionally, galantamine has anticonvulsant properties which may make it even more desirable as an antidote.
Research supported in part by the US Army has led to a US patent application for the use of galantamine and/or its derivatives for treatment of organophosphate poisoning. The indications for use of galantamine in the patent application include poisoning by nerve agents "including but not limited to soman, sarin, and VX, tabun, and Novichok agents". Galantamine was studied in the research cited in the patent application for use along with the well-recognized nerve agent antidote atropine. According to the investigators, an unexpected synergistic interaction occurred between galantamine and atropine in an amount of 6 mg/kg or higher. Increasing the dose of galantamine from 5 to 8 mg/kg decreased the dose of atropine needed to protect experimental animals from the toxicity of soman in dosages 1.5 times the dose generally required to kill half the experimental animals.
Autism
Galantamine given in addition to risperidone to autistic children has been shown to improve some of the symptoms of autism such as irritability, lethargy, and social withdrawal. Additionally, the cholinergic and nicotinic receptors are believed to play a role in attentional processes. Some studies have noted that cholinergic and nicotinic treatments have improved attention in autistic children. As such, it is hypothesized that galantamine's dual action mechanism might have a similar effect in treating autistic children and adolescents.
Anesthesia
Galantamine may have some limited use in reducing the side-effects of anesthetics ketamine and diazepam. In one study, a control group of patients were given ketamine and diazepam and underwent anesthesia and surgery. The experimental group was given ketamine, diazepam, and nivalin (of which the active ingredient is galantamine). The degree of drowsiness and disorientation of the two groups was then assessed 5, 10, 15, 30 and 60 minutes after surgery. The group that had taken nivalin were found to be more alert 5, 10, and 15 minutes after the surgery.
Oneirogen
Galantamine is known to have oneirogenic properties. Research has demonstrated its potential to increase dream recall, dream self-awareness and dream vividness. The enhancement of such dream properties can facilitate the induction of lucid dreams.
References
- "Galantamine Use During Pregnancy". Drugs.com. February 18, 2019. Archived from the original on February 25, 2020. Retrieved February 24, 2020.
- Anvisa (March 31, 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published April 4, 2023). Archived from the original on August 3, 2023. Retrieved August 16, 2023.
- "Razadyne- galantamine hydrobromide tablet, film coated". DailyMed. November 11, 2010. Archived from the original on April 26, 2023. Retrieved August 5, 2024.
- "Active substance: galantamine" (PDF). List of nationally authorised medicinal products, Human Medicines Evaluation Division. European Medicines Agency. November 12, 2020. Archived (PDF) from the original on October 31, 2021. Retrieved December 19, 2020.
- Theodorou M. "Sustainable Production of the Natural Product Galanthamine (Defra), NF0612". NNFCC Project Factsheet. The National Non-Food Crops Centre (NNFCC). Archived from the original on March 14, 2012.
- ^ "Galantamine". Drugs.com. August 8, 2023. Archived from the original on October 14, 2018. Retrieved March 26, 2024.
- ^ Birks J (January 2006). Birks JS (ed.). "Cholinesterase inhibitors for Alzheimer's disease". The Cochrane Database of Systematic Reviews. 2016 (1): CD005593. doi:10.1002/14651858.CD005593. PMC 9006343. PMID 16437532.
- ^ Kalola UK, Nguyen H (March 12, 2023). "Galantamine". StatPearls Publishing, US National Library of Medicine. PMID 34662060. Archived from the original on December 26, 2023. Retrieved March 26, 2024.
- Battle CE, Abdul-Rahim AH, Shenkin SD, Hewitt J, Quinn TJ (February 2021). "Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis". The Cochrane Database of Systematic Reviews. 2021 (2): CD013306. doi:10.1002/14651858.CD013306.pub2. PMC 8407366. PMID 33704781.
- Proskurnina NF, Areshknina LY. J. Chim. Gen. USSR. Chem. Abst. 1947;1948;1742(1595h):1216. No title available.
- Heinrich M (2004). "Snowdrops: The heralds of spring and a modern drug for Alzheimer's disease". Pharmaceutical Journal. 273 (7330): 905–6. OCLC 98892008. Archived from the original on October 23, 2018. Retrieved July 30, 2015.
- Mashkovsky MD, Kruglikova-Lvova RP (1951). "On the pharmacology of the new alkaloid galantamine". Farmakologia Toxicologia. 14: 27–30.
- Heinrich M, Lee Teoh H (June 2004). "Galanthamine from snowdrop--the development of a modern drug against Alzheimer's disease from local Caucasian knowledge". Journal of Ethnopharmacology. 92 (2–3): 147–162. doi:10.1016/j.jep.2004.02.012. PMID 15137996.
- Scott LJ, Goa KL (November 2000). "Galantamine: a review of its use in Alzheimer's disease". Drugs. 60 (5): 1095–1122. doi:10.2165/00003495-200060050-00008. PMID 11129124. S2CID 250305879.
- "Galantamine". alzforum. Archived from the original on November 17, 2019. Retrieved November 17, 2019.
- "Galantamine hydrobromide (trademark)" (PDF). US Food and Drug Administration. 2004. Archived (PDF) from the original on July 13, 2020. Retrieved December 17, 2017.
- Tricco AC, Soobiah C, Berliner S, Ho JM, Ng CH, Ashoor HM, et al. (November 2013). "Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysis". CMAJ. 185 (16): 1393–401. doi:10.1503/cmaj.130451. PMC 3826344. PMID 24043661.
- ^ Farlow MR (2003). "Clinical pharmacokinetics of galantamine". Clinical Pharmacokinetics. 42 (15): 1383–92. doi:10.2165/00003088-200342150-00005. PMID 14674789. S2CID 36855768.
- ^ Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV (April 2002). "Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial". Lancet. 359 (9314): 1283–90. doi:10.1016/S0140-6736(02)08267-3. PMID 11965273. S2CID 1172847.
- Hager K, Baseman AS, Nye JS, Brashear HR, Han J, Sano M, et al. (2014). "Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer's disease". Neuropsychiatric Disease and Treatment. 10: 391–401. doi:10.2147/NDT.S57909. PMC 3937252. PMID 24591834.
- "Alpha Cognition Announces FDA Acceptance of New Drug Application for ALPHA-1062 for Mild-to-Moderate Alzheimer's Disease" (Press release). Alpha Cognition. December 7, 2023. Archived from the original on April 16, 2024. Retrieved August 5, 2024 – via Business Wire.
- "Alpha Cognition's Oral Therapy Zunveyl Receives FDA Approval to Treat Alzheimer's Disease" (Press release). Alpha Cognition. July 29, 2024. Archived from the original on August 4, 2024. Retrieved August 4, 2024 – via Business Wire.
- ^ "Galantamine". Mayo Clinic. 2024. Archived from the original on March 26, 2024. Retrieved March 26, 2024.
- Birks J (January 2006). Birks J (ed.). "Cholinesterase inhibitors for Alzheimer's disease". The Cochrane Database of Systematic Reviews. 2016 (1): CD005593. doi:10.1002/14651858.CD005593. PMC 9006343. PMID 16437532.
- ^ Mannens GS, Snel CA, Hendrickx J, Verhaeghe T, Le Jeune L, Bode W, et al. (May 2002). "The metabolism and excretion of galantamine in rats, dogs, and humans". Drug Metabolism and Disposition. 30 (5): 553–63. doi:10.1124/dmd.30.5.553. PMID 11950787. S2CID 6795456.
- Woodruff-Pak DS, Vogel RW, Wenk GL (February 2001). "Galantamine: effect on nicotinic receptor binding, acetylcholinesterase inhibition, and learning". Proceedings of the National Academy of Sciences of the United States of America. 98 (4): 2089–94. Bibcode:2001PNAS...98.2089W. doi:10.1073/pnas.031584398. JSTOR 3055005. PMC 29386. PMID 11172080.
- Moerke MJ, McMahon LR, Wilkerson JL (April 2020). "More than Smoke and Patches: The Quest for Pharmacotherapies to Treat Tobacco Use Disorder". Pharmacological Reviews. 72 (2): 527–557. doi:10.1124/pr.119.018028. PMC 7090325. PMID 32205338. Archived from the original on October 8, 2021. Retrieved October 24, 2020.
- Kowal NM, Ahring PK, Liao WY, Indurti DC, Harvey BS, O'Connor SM, et al. (July 2018). "Galantamine is not a positive allosteric modulator of human α4β2 or α7 nicotinic acetylcholine receptors". British Journal of Pharmacology. 175 (14): 2911–2925. doi:10.1111/bph.14329. PMC 6016680. PMID 29669164.
- Greenblatt HM, Kryger G, Lewis T, Silman I, Sussman JL (December 1999). "Structure of acetylcholinesterase complexed with (-)-galanthamine at 2.3 A resolution". FEBS Letters. 463 (3): 321–6. Bibcode:1999FEBSL.463..321G. doi:10.1016/S0014-5793(99)01637-3. PMID 10606746. S2CID 573270.
- "Ortho-McNeil Neurologics, "Razadyne ER US Product Insert", May 2006" (PDF). Archived from the original (PDF) on December 22, 2009. Retrieved December 21, 2009.
- Kim JK, Park SU (2017). "Pharmacological aspects of galantamine for the treatment of Alzheimer's disease". EXCLI Journal. 16: 35–39. doi:10.17179/excli2016-820. PMC 5318685. PMID 28337117.
- Mucke HA (November 2015). "The case of galantamine: repurposing and late blooming of a cholinergic drug". Future Science OA. 1 (4): FSO73. doi:10.4155/fso.15.73. PMC 5137937. PMID 28031923.
- ^ Albuquerque EX, Pereira EF, Aracava Y, Fawcett WP, Oliveira M, Randall WR, et al. (August 2006). "Effective countermeasure against poisoning by organophosphorus insecticides and nerve agents". Proceedings of the National Academy of Sciences of the United States of America. 103 (35): 13220–5. Bibcode:2006PNAS..10313220A. doi:10.1073/pnas.0605370103. PMC 1550772. PMID 16914529.
- Albuquerque, Edson X, Adler, Michael, Pereira, Edna F.R. (January 22, 2009). "United States Patent Application 20090023706". US Patent and Trademark Office. Archived from the original on July 13, 2020. Retrieved May 27, 2016.
- Ghaleiha A, Ghyasvand M, Mohammadi MR, Farokhnia M, Yadegari N, Tabrizi M, et al. (July 2014). "Galantamine efficacy and tolerability as an augmentative therapy in autistic children: A randomized, double-blind, placebo-controlled trial". Journal of Psychopharmacology. 28 (7): 677–85. doi:10.1177/0269881113508830. PMID 24132248. S2CID 206491732.
- ^ Nicolson R, Craven-Thuss B, Smith J (October 2006). "A prospective, open-label trial of galantamine in autistic disorder". Journal of Child and Adolescent Psychopharmacology. 16 (5): 621–9. doi:10.1089/cap.2006.16.621. PMID 17069550.
- ^ Chakalova E, Marinova M, Srebreva M, Anastasov D, Ploskov K (1987). "". Akusherstvo I Ginekologiia (in Bulgarian). 26 (3): 28–31. PMID 3631427.
- Tan S, Fan J (June 2023). "A systematic review of new empirical data on lucid dream induction techniques". Journal of Sleep Research. 32 (3): e13786. doi:10.1111/jsr.13786. PMID 36408823.
- Sparrow G, Hurd R, Carlson R, Molina A (August 2018). "Exploring the effects of galantamine paired with meditation and dream reliving on recalled dreams: Toward an integrated protocol for lucid dream induction and nightmare resolution". Consciousness and Cognition. 63. Elsevier BV: 74–88. doi:10.1016/j.concog.2018.05.012. PMID 29960246.
| Psychoanaleptics: Anti-dementia agents (ATC code N06D and others) | |
|---|---|
| AChE inhibitor medications | |
| Other medications | |
| Experimental BACE inhibitors | |
| Nicotinic acetylcholine receptor modulators | |||||
|---|---|---|---|---|---|
| nAChRsTooltip Nicotinic acetylcholine receptors |
| ||||
| Precursors (and prodrugs) | |||||