| |
| |
| |
| Names | |
|---|---|
| IUPAC names
Uranium hexafluoride Uranium(VI) fluoride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | hex |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.116 |
| EC Number |
|
| Gmelin Reference | 2923 |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 2978 (<1% U) 2977 (>1% U) |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
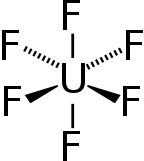
| Chemical formula | UF6 |
| Molar mass | 352.02 g/mol |
| Appearance | Colorless solid |
| Density | 5.09 g/cm, solid |
| Boiling point | 56.5 °C (133.7 °F; 329.6 K) (sublimes, at atmospheric pressure) |
| Solubility in water | Hydrolyzes |
| Solubility |
|
| Structure | |
| Crystal structure | Orthorhombic, oP28 |
| Space group | Pnma, No. 62 |
| Coordination geometry | Octahedral (Oh) |
| Dipole moment | 0 |
| Thermochemistry | |
| Std molar entropy (S298) |
|
| Std enthalpy of formation (ΔfH298) |
|
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Toxic, corrosive, radioactive |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H300, H330, H373, H411 |
| NFPA 704 (fire diamond) |
 OX |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | ICSC 1250 |
| Related compounds | |
| Other anions | Uranium hexachloride |
| Other cations | |
| Related uranium fluorides | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Uranium hexafluoride, sometimes called hex, is an inorganic compound with the formula UF6. Uranium hexafluoride is a volatile, toxic white solid that is used in the process of enriching uranium, which produces fuel for nuclear reactors and nuclear weapons.
Preparation
Uranium dioxide is converted with hydrofluoric acid (HF) to uranium tetrafluoride:
- UO2 + 4 HF → UF4 + 2 H2O
In samples contaminated with uranium trioxide, the oxyfluoride is produced in the HF step:
- UO3 + HF → UF2O2 + H2O
The resulting UF4 is subsequently oxidized with fluorine to give the hexafluoride:
- UF4 + F2 → UF6
Properties
Physical properties
At atmospheric pressure, UF6 sublimes at 56.5 °C.

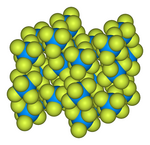
The solid-state structure was determined by neutron diffraction at 77 K and 293 K.
-
 Ball-and-stick model of the unit cell of uranium hexafluoride
Ball-and-stick model of the unit cell of uranium hexafluoride
-
 Bond lengths and angles of gaseous uranium hexafluoride
Bond lengths and angles of gaseous uranium hexafluoride
Chemical properties
UF6 reacts with water, releasing hydrofluoric acid. The compound reacts with aluminium, forming a surface layer of AlF3 that resists any further reaction from the compound.
Uranium hexafluoride is a mild oxidant. It is a Lewis acid as evidenced by its binding to form heptafluorouranate(VI), [UF7].
Polymeric uranium(VI) fluorides containing organic cations have been isolated and characterized by X-ray diffraction.
Application in the fuel cycle
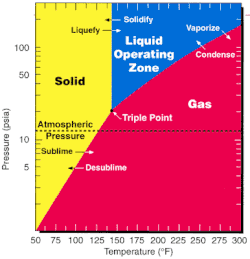
As one of the most volatile compounds of uranium, uranium hexafluoride is relatively convenient to process and is used in both of the main uranium enrichment methods, namely gaseous diffusion and the gas centrifuge method. Since the triple point of UF6; 64 °C(147 °F; 337 K) and 152 kPa (22 psi; 1.5 atm); is close to ambient conditions, phase transitions can be achieved with little thermodynamic work.
Fluorine has only a single naturally occurring stable isotope, so isotopologues of UF6 differ in their molecular weight based solely on the uranium isotope present. This difference is the basis for the physical separation of isotopes in enrichment.
All the other uranium fluorides are nonvolatile solids that are coordination polymers.
The conversion factor for the U isotopologue of UF6 ("hex") to "U mass" is 0.676.
Gaseous diffusion requires about 60 times as much energy as the gas centrifuge process: gaseous diffusion-produced nuclear fuel produces 25 times more energy than is used in the diffusion process, while centrifuge-produced fuel produces 1,500 times more energy than is used in the centrifuge process.
In addition to its use in enrichment, uranium hexafluoride has been used in an advanced reprocessing method (fluoride volatility), which was developed in the Czech Republic. In this process, spent nuclear fuel is treated with fluorine gas to transform the oxides or elemental metals into a mixture of fluorides. This mixture is then distilled to separate the different classes of material. Some fission products form nonvolatile fluorides which remain as solids and can then either be prepared for storage as nuclear waste or further processed either by solvation-based methods or electrochemically.
Uranium enrichment produces large quantities of depleted uranium hexafluoride (DUF6 or D-UF6) as a waste product. The long-term storage of D-UF6 presents environmental, health, and safety risks because of its chemical instability. When UF6 is exposed to moist air, it reacts with the water in the air to produce UO2F2 (uranyl fluoride) and HF (hydrogen fluoride) both of which are highly corrosive and toxic. In 2005, 686,500 tonnes of D-UF6 was housed in 57,122 storage cylinders located near Portsmouth, Ohio; Oak Ridge, Tennessee; and Paducah, Kentucky. Storage cylinders must be regularly inspected for signs of corrosion and leaks. The estimated lifetime of the steel cylinders is measured in decades.
Accidents and disposal
There have been several accidents involving uranium hexafluoride in the US, including a cylinder-filling accident and material release at the Sequoyah Fuels Corporation in 1986 where an estimated 29 500 pounds of gaseous UF6 escaped. The U.S. government has been converting DUF6 to solid uranium oxides for disposal. Such disposal of the entire DUF6 stockpile could cost anywhere from $15 million to $450 million.
-
 Ruptured 14-ton UF6 shipping cylinder. 1 fatality, dozens injured. ~29500 lbs of material released. Sequoyah Fuels Corporation 1986.
Ruptured 14-ton UF6 shipping cylinder. 1 fatality, dozens injured. ~29500 lbs of material released. Sequoyah Fuels Corporation 1986.
-
 DUF6 storage yard from afar
DUF6 storage yard from afar
-
 DUF6 cylinders: painted (left) and corroded (right)
DUF6 cylinders: painted (left) and corroded (right)
References
- "Uranium Hexafluoride". Archived from the original on 2013-09-16. Retrieved 2013-08-08.
- ^ Johnson, Gerald K. (1979). "The Enthalpy of Formation of Uranium Hexafluoride". The Journal of Chemical Thermodynamics. 11 (5): 483–490. doi:10.1016/0021-9614(79)90126-5.
- Uranium(VI) fluoride
- ^ Peehs, Martin; Walter, Thomas; Walter, Sabine; Zemek, Martin (2007). "Uranium, Uranium Alloys, and Uranium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a27_281.pub2. ISBN 978-3-527-30385-4.
- Brickwedde, Ferdinand G.; Hoge, Harold J.; Scott, Russell B. (1948). "The Low Temperature Heat Capacities, Enthalpies, and Entropies of UF4 and UF6". J. Chem. Phys. 16 (5): 429–436. Bibcode:1948JChPh..16..429B. doi:10.1063/1.1746914.
- J. H. Levy; John C. Taylor; Paul W. Wilson (1976). "Structure of Fluorides. Part XII. Single-Crystal Neutron Diffraction Study of Uranium Hexafluoride at 293 K". J. Chem. Soc., Dalton Trans. (3): 219–224. doi:10.1039/DT9760000219.
- J. H. Levy, J. C. Taylor and A. B. Waugh (1983). "Neutron Powder Structural Studies of UF6, MoF6 and WF6 at 77 K". Journal of Fluorine Chemistry. 23: 29–36. doi:10.1016/S0022-1139(00)81276-2.
- J. C. Taylor, P. W. Wilson, J. W. Kelly: „The structures of fluorides. I. Deviations from ideal symmetry in the structure of crystalline UF6: a neutron diffraction analysis", Acta Crystallogr., 1973, B29, p. 7–12; doi:10.1107/S0567740873001895.
- Kimura, Masao; Schomaker, Werner; Smith, Darwin W.; Bernard (1968). "Electron-Diffraction Investigation of the Hexafluorides of Tungsten, Osmium, Iridium, Uranium, Neptunium, and Plutonium". J. Chem. Phys. 48 (8): 4001–4012. Bibcode:1968JChPh..48.4001K. doi:10.1063/1.1669727. Archived from the original on 2023-01-11. Retrieved 2020-10-10.
- G. H. Olah; J. Welch (1978). "Synthetic methods and reactions. 46. Oxidation of organic compounds with uranium hexafluoride in haloalkane solutions". J. Am. Chem. Soc. 100 (17): 5396–5402. doi:10.1021/ja00485a024.
- J. A. Berry; R. T. Poole; A. Prescott; D. W. A. Sharp; J. M. Winfield (1976). "The oxidising and fluoride ion acceptor properties of uranium hexafluoride in acetonitrile". J. Chem. Soc., Dalton Trans. (3): 272–274. doi:10.1039/DT9760000272.
- S. M. Walker; P. S. Halasyamani; S. Allen; D. O'Hare (1999). "From Molecules to Frameworks: Variable Dimensionality in the UO2(CH3COO)2·2H2O/HF(aq)/Piperazine System. Syntheses, Structures, and Characterization of Zero-Dimensional (C4N2H12)UO2F4·3H2O, One-Dimensional (C4N2H12)2U2F12·H2O, Two-Dimensional (C4N2H12)2(U2O4F5)4·11H2O, and Three-Dimensional (C4N2H12)U2O4F6". J. Am. Chem. Soc. 121 (45): 10513–10521. doi:10.1021/ja992145f.
- "Uranium Hexafluoride: Source: Appendix A of the PEIS (DOE/EIS-0269): Physical Properties". web.evs.anl.gov. Retrieved 2022-08-18.
- "Uranium Enrichment and the Gaseous Diffusion Process". USEC Inc. Archived from the original on 2007-10-19. Retrieved 2007-09-24.
- "Unit converter molar mass calculator". TranslatorsCafé. Mississauga, Ontario, Canada: ANVICA Software Development. 1 February 2021.
- "How much depleted uranium hexafluoride is stored in the United States?". Depleted UF6 FAQs. Argonne National Laboratory.
- "Depleted UF6 Management Program Documents". Archived from the original on 2008-02-16. Retrieved 2006-05-17.
- "What is DUF6? Is it dangerous and what should we do with it?". Institute for Energy and Environmental Research. 2007-09-24.
- Brugge, D.; Delemos, J. L.; Bui, C. (2007). "The Sequoyah Corporation Fuels Release and the Church Rock Spill: Unpublicized Nuclear Releases in American Indian Communities". American Journal of Public Health. 97 (9): 1595–1600. doi:10.2105/AJPH.2006.103044. PMC 1963288. PMID 17666688.
- "Have there been accidents involving uranium hexafluoride?". Depleted UF6 FAQs. Argonne National Laboratory. Archived from the original on 2017-06-09.
- "What is going to happen to the uranium hexafluoride stored in the United States?". Depleted UF6 FAQs. Argonne National Laboratory.
- "Are there any currently-operating disposal facilities that can accept all of the depleted uranium oxide that would be generated from conversion of DOE's depleted UF6 inventory?". Depleted UF6 FAQs. Argonne National Laboratory.
Further reading
- Gmelins Handbuch der anorganischen Chemie, System Nr. 55, Uran, Teil A, p. 121–123.
- Gmelins Handbuch der anorganischen Chemie, System Nr. 55, Uran, Teil C 8, p. 71–163.
- R. DeWitt: Uranium hexafluoride: A survey of the physico-chemical properties, Technical report, GAT-280; Goodyear Atomic Corp., Portsmouth, Ohio; 12. August 1960; doi:10.2172/4025868.
- Ingmar Grenthe, Janusz Drożdżynński, Takeo Fujino, Edgar C. Buck, Thomas E. Albrecht-Schmitt, Stephen F. Wolf: Uranium Archived 2012-01-18 at the Wayback Machine, in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Hrsg.): The Chemistry of the Actinide and Transactinide Elements, Springer, Dordrecht 2006; ISBN 1-4020-3555-1, p. 253–698; doi:10.1007/1-4020-3598-5_5 (p. 530–531, 557–564).
- US-Patent 2535572: Preparation of UF6; 26. December 1950.
- US-Patent 5723837: Uranium Hexafluoride Purification; 3. March 1998.
External links
- Simon Cotton (Uppingham School, Rutland, UK): Uranium Hexafluoride.
- Uranium Hexafluoride (UF6) – Physical and chemical properties of UF6, and its use in uranium processing – Uranium Hexafluoride and Its Properties
- Uranium Hexafluoride at WebElements
- Import of Western depleted uranium hexafluoride (uranium tails) to Russia
| Binary hexafluorides | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Known binary hexafluorides |
| ||||||||
| Predicted binary hexafluorides |
| ||||||||
| Uranium compounds | |||
|---|---|---|---|
| U(II) | |||
| U(III) |
| ||
| U(IV) |
| ||
| U(IV,V) | |||
| U(IV,VI) | |||
| U(V) | |||
| U(VI) | |||
| U(XII) |
| ||
| Halides of actinides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||