| |
| Names | |
|---|---|
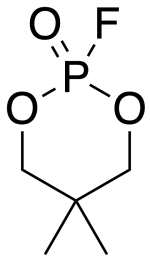
| Preferred IUPAC name 2-Fluoro-5,5-dimethyl-1,3,2λ-dioxaphosphinan-2-one | |
| Other names NPF, neopentylene phosphoryl fluoridate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H10FO3P |
| Molar mass | 168.104 g·mol |
| Melting point | 41–42 °C (106–108 °F; 314–315 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Neopentylene fluorophosphate, also known as NPF, is an organophosphate compound that is classified as a nerve agent. It has a comparatively low potency, but is stable and persistent, with a delayed onset of action and long duration of effects.
See also
References
- Edmundson R. Dictionary of Organophosphorus Compounds. pp 435-436. ISBN 9780412257902
- Hart GJ, O'Brien RD, Milbrath DS, Verkade JG (1976). "Dissociation and phosphorylation constants for the inhibition of acetylcholinesterase by 2-fluoro, 2-oxo-1,3,2-dioxaphosphorinanes". Pesticide Biochemistry and Physiology. 6 (5): 464–470. doi:10.1016/0048-3575(76)90057-2. ISSN 0048-3575.
- Crippin JB (2006). Explosives and Chemical Weapons Identification. Taylor & Francis. p. 21. ISBN 978-0-8493-3338-5.
- Ledgard J (2006). A laboratory history of chemical warfare agents (2nd ed.). ISBN 978-0-6151-3645-5.
| Neurotoxins | |
|---|---|
| Animal toxins | |
| Bacterial | |
| Cyanotoxins | |
| Plant toxins | |
| Mycotoxins | |
| Pesticides | |
| Nerve agents | |
| Bicyclic phosphates | |
| Cholinergic neurotoxins | |
| Psychoactive drugs | |
| Other | |
| Acetylcholine metabolism and transport modulators | |||||||
|---|---|---|---|---|---|---|---|
| Enzyme (modulators) |
| ||||||
| Transporter (modulators) |
| ||||||
| Release (modulators) |
| ||||||
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |