| Revision as of 18:53, 16 February 2024 editKeresluna (talk | contribs)Autopatrolled, Extended confirmed users4,074 edits →External links: replace template← Previous edit | Latest revision as of 03:58, 28 November 2024 edit undoDMacks (talk | contribs)Edit filter managers, Autopatrolled, Administrators186,553 edits MOS | ||
| Line 33: | Line 33: | ||
| | Appearance = orange-yellow ] crystals | | Appearance = orange-yellow ] crystals | ||
| | Density = 6.75 g/cm<sup>3</sup> | | Density = 6.75 g/cm<sup>3</sup> | ||
| | MeltingPt = sublimes above 300°C | | MeltingPt = sublimes above 300 °C | ||
| | BoilingPt = | | BoilingPt = | ||
| | Solubility = Reacts<ref name="fluoride">{{cite journal |author1=Victor Lenher |title=Fluoride of Gold |
| Solubility = Reacts<ref name="fluoride">{{cite journal |author1=Victor Lenher |title=Fluoride of Gold |journal=Journal of the American Chemical Society |date=1903 |volume=25 |issue=11 |pages=1136–1138 |doi=10.1021/ja02013a004 |url=https://zenodo.org/record/1707291 |language=en}}</ref><ref name="pre" /> | ||
| | MagSus = +74·10<sup>−6</sup> cm<sup>3</sup>/mol }} | | MagSus = +74·10<sup>−6</sup> cm<sup>3</sup>/mol }} | ||
| |Section3={{Chembox Structure | |Section3={{Chembox Structure | ||
| Line 58: | Line 58: | ||
| == Preparation == | == Preparation == | ||
| AuF<sub>3</sub> can be prepared by reacting ] with ] or ].<ref name="pre">{{cite journal |author1=Inis C. Tornieporth-Oetting |author2=Thomas M. Klapötke |title=Laboratory Scale Direct Synthesis of Pure |
AuF<sub>3</sub> can be prepared by reacting ] with ] or ].<ref name="pre">{{cite journal |author1=Inis C. Tornieporth-Oetting |author2=Thomas M. Klapötke |title=Laboratory Scale Direct Synthesis of Pure AuF<sub>3</sub> |journal=Chemische Berichte |date=1995 |volume=128 |issue=9 |pages=957–958 |doi=10.1002/cber.19951280918 |language=en}}</ref> | ||
| == Structure == | == Structure == | ||
Latest revision as of 03:58, 28 November 2024
For other uses, see Gold fluoride.
| |
| Names | |
|---|---|
| IUPAC name Gold(III) fluoride | |
| Other names
Gold trifluoride Auric fluoride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | AuF3 |
| Molar mass | 253.961779 g·mol |
| Appearance | orange-yellow hexagonal crystals |
| Density | 6.75 g/cm |
| Melting point | sublimes above 300 °C |
| Solubility in water | Reacts |
| Magnetic susceptibility (χ) | +74·10 cm/mol |
| Structure | |
| Crystal structure | Hexagonal, hP24 |
| Space group | P6122, No. 178 |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH298) |
-363.3 kJ/mol |
| Related compounds | |
| Other anions | Gold(III) chloride Gold(III) bromide |
| Other cations | Silver fluoride Copper(II) fluoride Mercury(II) fluoride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Gold(III) fluoride, AuF3, is an orange solid that sublimes at 300 °C. It is a powerful fluorinating agent. It is very sensitive to moisture, yielding gold(III) hydroxide and hydrofluoric acid.
Preparation
AuF3 can be prepared by reacting AuCl3 with F2 or BrF3.
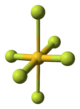
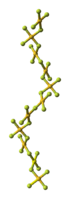
Structure
The crystal structure of AuF3 consists of spirals of square-planar AuF4 units.
 |
 |
 |
 |
|
| AuF3 unit cell | neighbouring (AuF3)n helices | distorted octahedral coordination of gold by six fluorines | top-down view of an (AuF3)n helix | side view of an (AuF3)n helix |
References
- Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 4–59. ISBN 0-8493-0594-2.
- Victor Lenher (1903). "Fluoride of Gold". Journal of the American Chemical Society. 25 (11): 1136–1138. doi:10.1021/ja02013a004.
- ^ Inis C. Tornieporth-Oetting; Thomas M. Klapötke (1995). "Laboratory Scale Direct Synthesis of Pure AuF3". Chemische Berichte. 128 (9): 957–958. doi:10.1002/cber.19951280918.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8., p. 1184.
- F. W. B. Einstein; P. R. Rao; James Trotter; Neil Bartlett (1967). "The crystal structure of gold trifluoride". Journal of the Chemical Society A: Inorganic, Physical, Theoretical. 4: 478–482. doi:10.1039/J19670000478.
External links
 Media related to Gold trifluoride at Wikimedia Commons
Media related to Gold trifluoride at Wikimedia Commons
| Gold compounds | |||
|---|---|---|---|
| Gold(-I) | |||
| Gold(I) |
| ||
| Gold(II) | |||
| Gold(I,III) | |||
| Gold(III) |
| ||
| Gold(V) | |||
| Gold(VI) |
| ||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |