This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 15:58, 11 February 2011 (Updating {{drugbox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Misplaced Pages talk:WikiProject_Pharmacology|errors). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 15:58, 11 February 2011 by CheMoBot (talk | contribs) (Updating {{drugbox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Misplaced Pages talk:WikiProject_Pharmacology|errors)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound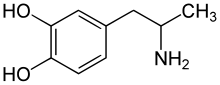 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C9H13NO2 |
| Molar mass | 167,21 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
α-Methyldopamine (α-Me-DA), also known as 3,4-dihydroxyamphetamine (3,4-DHA), is a neurotoxin and research chemical of the phenethylamine and amphetamine chemical classes. It is a metabolite of various amphetamine derivatives such as amphetamine itself, methamphetamine, and MDMA ("Ecstasy"), MDA and has been suggested to play significant role in their neurotoxic effects on monoaminergic neurons. Further study has suggested that, alpha-methyldopamine itself is not directly responsible for the damaging effects, but a specific reaction between alpha-methyldopamine and one of the bodies endogenous antioxidants -glutathione, may produce 2,5-Bis-(glutathion-S-yl)-alpha-methyldopamine, which has been demonstrated in laboratory animals to cause the same neurotoxic effects seen by related stimulants and empathogens.
The basis of this idea is in the observation that MDA and MDMA may not themselves be responsible for their neurotoxicity, as an intracerebroventricular injection (injection directly into the brain itself) does not appear to cause neurotoxicity. While many studies cite excitotoxicity, or oxidative stress as likely mechanisms, which may be an effect of the chemical itself, this has led to the search for other mechanisms for the observed toxicity of serotonin neurons and subsequent reduction in 5-HT (Serotonin) and 5-HIAA (its major metabolite in the body) in vivo following administration. A common theory follows that a metabolite in the periphery must be responsible, and several have been cited as responsible. Although, alpha-methyldopamine is widely cited as the source of this neurotoxicity in a number of lay sources, McCann, et al. (1991), demonstrated that the major metabolites alpha-methyldopamine (α-MeDA) and 3-O-methyl-α-methyldopamine (3-O-Me-α-MeDA) did not produce neurotoxicity.
It was first demonstrated, in 1978, by Conway, et al. and possibly others that, while alpha-methyldopamine caused acute decreases in the levels of neuronal dopamine, in some areas of the brain in excess of 75%, levels returned to baseline within 12 hours, indicating that alpha-methyldopamine would not be responsible for the toxic effects observed.
However, the story complicates as alpha-methyldopamine readily oxidizes to the o-quinoneand reacts with endogenous antioxidants in the body, such as glutathione (GSH). The mechanism of action behind amphetamines and their related compounds, causes the normally encapsulated neurotransmitters to be released from their oxidative vesicle within the neuron into the reductive environment of the cell cystol. This sudden release of oxidative molecules into the cell puts it under oxidative stress. As Glutathione is the major endogenous anti-oxidant produced in the body to protect against oxidative stress and xenobiotics (foreign compounds), it likely plays at least some role in the neutralization of the suddenly released catecholamines (Dopamine and Norepinephrine), and Serotonin, as well as the break down of amphetamine, methamphetamine, MDA, MDMA, and other MDxx compounds. It was demonstrated by Miller, et al. (1997), that 5-(glutathion-S-yl)-alpha-methyldopamine and 5-(N-acetylcystein-S-yl)-alpha-methyldopamine produced similar effects to the parent compound, but did not induce neurotoxicity. Another related compound however, 2,5-bis-(glutathion-S-yl)-alpha-methyldopamine, did in fact induce neurotoxicity, providing initial evidence that this metabolite may be the source of neuronal toxicity following the administration of MDA and MDMA, and the subsequent reduction in 5-HT (Serotonin) axons..
See also
References
- McCann, Una D.; Ricaurte, George A. (5 April 1991.), "Major metabolites of(±)3,4-methylenedioxyamphetamine (MDA) do not mediate its toxic effects on brain serotonin neurons.", Brain Research, Volume 545 (Issue 1-2.): Pages 279–282., doi:doi:10.1016/0006-8993(91)91297-E
{{citation}}:|issue=has extra text (help);|volume=has extra text (help); Check|doi=value (help); Check date values in:|date=(help) - Conway, EL; Louis, WJ; Jarrot, B. (1 Dec. 1978), "Acute and chronic administration of alpha-methyldopa: regional levels of endogenous and alpha-methylated catecholamines in rat brain.", Eur J Pharmacol., Volume 52. (Issue 3-4.): 271–80., PMID 729639
{{citation}}:|issue=has extra text (help);|volume=has extra text (help); Check date values in:|date=(help) - Miller, RT; Lau, SS; Monks, TJ (1997 Apr 4.), "2,5-Bis-(glutathion-S-yl)-alpha-methyldopamine, a putative metabolite of (+/-)-3,4-methylenedioxyamphetamine, decreases brain serotonin concentrations.", Eur J Pharmacol., Volume 323. (Issue 2-3.): 173–80, PMID: 9128836
{{citation}}:|issue=has extra text (help);|volume=has extra text (help); Check date values in:|date=(help)
| Dopamine receptor modulators | |||||||
|---|---|---|---|---|---|---|---|
| D1-like |
| ||||||
| D2-like |
| ||||||
| Phenethylamines | |
|---|---|
| Phenethylamines |
|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|