| Revision as of 20:24, 29 June 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (changes to watched fields - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Wikipe← Previous edit | Latest revision as of 08:59, 22 January 2024 edit undoOndraMix (talk | contribs)33 editsm typoTag: Visual edit | ||
| (32 intermediate revisions by 20 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | | Watchedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 436917297 | ||
| | |
| Name = Niobium(V) fluoride | ||
| | |
| ImageFile = Niobium(V) fluoride.png | ||
| | ImageSize = 300px | |||
| | ImageName = | |||
| | |
| ImageName = | ||
| | ImageFile1 = | |||
| | |
| IUPACName = Niobium(V) fluoride<br/>Niobium pentafluoride | ||
| | |
|Section1={{Chembox Identifiers | ||
| | CASNo = 7783-68-8 | |||
| | |
| CASNo_Ref = {{cascite|correct|??}} | ||
| | |
| CASNo = 7783-68-8 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | |
||
| | |
| UNII = T86H76439H | ||
| | PubChem = 82217 | |||
| ⚫ | | EINECS = 232-020-2 | ||
| | RTECS = | |||
| | ChemSpiderID = 74197 | |||
| | StdInChI=1S/5FH.Nb/h5*1H;/q;;;;;+5/p-5 | |||
| | StdInChIKey = AOLPZAHRYHXPLR-UHFFFAOYSA-I | |||
| | SMILES = F(F)(F)(F)F | |||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| |Nb=1|F=5 | |||
| | Formula = NbF<sub>5</sub> | |||
| | |
| Appearance = colorless ] solid | ||
| | |
| MolarMass = | ||
| | |
| Density = 3.293 g/cm<sup>3</sup> | ||
| | |
| Solubility = reacts | ||
| | |
| SolubleOther = slightly soluble in ], ], ] | ||
| | |
| MeltingPtC = 72 to 73 | ||
| | MeltingPt_notes = | |||
| | BoilingPt = 236 °C | |||
| | BoilingPtC = 236 | |||
| }} | }} | ||
| | |
|Section4={{Chembox Thermochemistry | ||
| | |
| DeltaHf = | ||
| | |
| Entropy = | ||
| }} | }} | ||
| | |
|Section7={{Chembox Hazards | ||
| | |
| ExternalSDS = | ||
| ⚫ | | FlashPt = Non-flammable | ||
| | EUIndex = Not listed | |||
| ⚫ | | PEL = | ||
| ⚫ | | |
||
| | GHSPictograms = {{GHS05}}{{GHS07}} | |||
| ⚫ | | |
||
| | GHSSignalWord = Warning | |||
| | HPhrases = {{H-phrases|302|312|314|332}} | |||
| | PPhrases = {{P-phrases|260|261|264|270|271|280|301+312|301+330+331|302+352|303+361+353|304+312|304+340|305+351+338|310|312|321|322|330|363|405|501}} | |||
| }} | }} | ||
| | |
|Section8={{Chembox Related | ||
| | |
| OtherAnions = ]<br/>]<br/>] | ||
| | |
| OtherCations = ]<br/>] | ||
| | |
| OtherFunction = ]<br/>] | ||
| | |
| OtherFunction_label = niobium fluorides | ||
| }} | }} | ||
| }} | }} | ||
| '''Niobium(V) fluoride''', also known as '''niobium pentafluoride''', is a colorless |
'''Niobium(V) fluoride''', also known as '''niobium pentafluoride''', is the ] with the formula NbF<sub>5</sub>. It is a colorless solid.<ref name=Ullmann>{{cite encyclopedia|author1=Joachim Eckert |author2=Hermann C. Starck|title=Niobium and Niobium Compounds|encyclopedia=Ullmann's Encyclopedia of Industrial Chemistry|year=2005|publisher=Wiley-VCH|place=Weinheim|doi=10.1002/14356007.a17_251|isbn=3527306730}}</ref> | ||
| ==Preparation== | ==Preparation and structure== | ||
| Niobium pentafluoride is obtained by treatment of any niobium compound with fluorine:<ref>{{cite book|chapter=Anhydrous Metal Fluorides|author=Homer F. Priest|title=Inorganic Syntheses |year=1950|volume=3|page=171|doi=10.1002/9780470132340.ch47}}</ref> | |||
| Niobium pentafluoride is obtained as an intermediate during the recovery of ] metal from its ores. It also can be prepared by direct fluorination of niobium metal at 250 to 300°C, either by ] gas or anhydrous ]. The pentafluoride vapors are condensed in a ] or ] tube from which it is sublimed at 120°C under vacuum and collected as colorless crystals. | |||
| ⚫ | :2 Nb + 5 F<sub>2</sub> → 2 NbF<sub>5</sub> | ||
| :2 NbCl<sub>5</sub> + 5 F<sub>2</sub> → 2 NbF<sub>5</sub> + 5 Cl<sub>2</sub> | |||
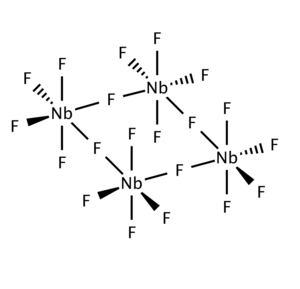
| As shown by ], the solid consists of tetramers <sub>4</sub>. This structure is related to that for WOF<sub>4</sub>.<ref>{{cite journal |doi=10.1039/jr9640003714 |title=717. The structures of niobium and tantalum pentafluorides |year=1964 |last1=Edwards |first1=A. J. |journal=Journal of the Chemical Society (Resumed) |page=3714 }}</ref> | |||
| Also, niobium pentafluroide can be prepared by the reaction of fluorine with ]: | |||
| ⚫ | : |
||
| ==Reactions== | |||
| It reacts with ] to give H<sub>2</sub>NbF<sub>7</sub>, a ]. In ], NbF<sub>5</sub> converts to <sup>2-</sup> and <sup>2-</sup>. The relative solubility of {{chem2|K2}} (M = Nb, Ta) is the basis of the ] for separation of Nb and Ta. | |||
| ] forms a dimeric structure (edge-shared bioctahedron) in contrast to the corner-shared tetrameric structure of the fluoride. | |||
| ==External links== | ==External links== | ||
| * | * | ||
| ==References== | |||
| <references /> | |||
| {{Niobium compounds}} | {{Niobium compounds}} | ||
| {{fluorine compounds}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
Latest revision as of 08:59, 22 January 2024
| |
| Names | |
|---|---|
| IUPAC names
Niobium(V) fluoride Niobium pentafluoride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.109 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | F5Nb |
| Molar mass | 187.89839 g·mol |
| Appearance | colorless hygroscopic solid |
| Density | 3.293 g/cm |
| Melting point | 72 to 73 °C (162 to 163 °F; 345 to 346 K) |
| Boiling point | 236 °C (457 °F; 509 K) |
| Solubility in water | reacts |
| Solubility | slightly soluble in chloroform, carbon disulfide, sulfuric acid |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Warning |
| Hazard statements | H302, H312, H314, H332 |
| Precautionary statements | P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P363, P405, P501 |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Niobium(V) chloride Niobium(V) bromide Niobium(V) iodide |
| Other cations | Vanadium(V) fluoride Tantalum(V) fluoride |
| Related niobium fluorides | Niobium(III) fluoride Niobium(IV) fluoride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Niobium(V) fluoride, also known as niobium pentafluoride, is the inorganic compound with the formula NbF5. It is a colorless solid.
Preparation and structure
Niobium pentafluoride is obtained by treatment of any niobium compound with fluorine:
- 2 Nb + 5 F2 → 2 NbF5
- 2 NbCl5 + 5 F2 → 2 NbF5 + 5 Cl2
As shown by X-ray crystallography, the solid consists of tetramers 4. This structure is related to that for WOF4.
Reactions
It reacts with hydrogen fluoride to give H2NbF7, a superacid. In hydrofluoric acid, NbF5 converts to and . The relative solubility of K2[MFO] (M = Nb, Ta) is the basis of the Marignac process for separation of Nb and Ta.
NbCl5 forms a dimeric structure (edge-shared bioctahedron) in contrast to the corner-shared tetrameric structure of the fluoride.
External links
References
- Joachim Eckert; Hermann C. Starck (2005). "Niobium and Niobium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_251. ISBN 3527306730.
- Homer F. Priest (1950). "Anhydrous Metal Fluorides". Inorganic Syntheses. Vol. 3. p. 171. doi:10.1002/9780470132340.ch47.
- Edwards, A. J. (1964). "717. The structures of niobium and tantalum pentafluorides". Journal of the Chemical Society (Resumed): 3714. doi:10.1039/jr9640003714.
| Niobium compounds | |||
|---|---|---|---|
| Niobium(II) | |||
| Niobium(III) | |||
| Niobium(IV) | |||
| Niobium(V) |
| ||