| Revision as of 13:38, 7 March 2007 edit769z (talk | contribs)13 edits →Effects← Previous edit | Latest revision as of 00:31, 8 January 2025 edit undoRunningman12345 (talk | contribs)7 edits →Synthesis: erowid is not a peer reviewed scientific articleTag: references removed | ||
| Line 1: | Line 1: | ||
| {{Short description|Hallucinogenic drug}} | |||
| {{otheruses}} | |||
| {{cs1 config|name-list-style=vanc|display-authors=6}} | |||
| {{drugbox | | |||
| {{Redirect-distinguish|Lsd|£sd}} | |||
| | width = 300 | |||
| {{other uses}} | |||
| | image = LSD-2D, 3D.png | |||
| {{Use mdy dates|date=May 2016}} | |||
| | IUPAC_name = (6a''R'',9''R'')-''N'',''N''-diethyl-7-methyl-4,6,6a,7,8,9-<br>hexahydroindolo-quinoline-9-carboxamide | |||
| {{Infobox drug | |||
| | CAS_number = 50-37-3 | |||
| | Watchedfields = changed | |||
| | PubChem = 5761 | |||
| | verifiedrevid = 629704081 | |||
| | C=20 | H=25| N=3| O=1 | |||
| | drug_name = Lysergic acid diethylamide | |||
| | molecular_weight = 323.43 g/mol | |||
| | INN = Lysergide | |||
| | melting_point = 80 | |||
| | type = | |||
| | synonyms = LSD, LSD-25, lysergide, d-lysergic acid diethylamide, N,N-diethyl-d-lysergamide | |||
| | IUPAC_name = (6a''R'',9''R'')-''N'',''N''-diethyl-7-methyl-4,6,6a,7,8,9-hexahydroindoloquinoline-9-carboxamide | |||
| | smiles = CN1(C2=C | |||
| | image = LSD Structure V2.svg | |||
| (C(N(CC)CC)=O)C1)() | |||
| | alt = | |||
| CC3=CNC4=C3C2=CC=C4 | |||
| | caption = ] of LSD | |||
| | elimination_half-life = 3 hours | |||
| | caption2 = 3D stick model of LSD | |||
| | metabolism = hepatic | |||
| | Drugs.com = | |||
| | excretion = renal | |||
| | |
| pregnancy_US = C | ||
| | |
| MedlinePlus = | ||
| | |
| licence_EU = | ||
| | licence_US = | |||
| | pregnancy_AU = | |||
| | pregnancy_category = C | |||
| | tradename = Delysid | |||
| | legal_AU = Schedule 9 | | legal_AU = Schedule 9 | ||
| | |
| legal_BR = F2 | ||
| | legal_CA = Schedule III | |||
| | legal_NZ = Class A | |||
| | legal_UK = Class A | | legal_UK = Class A | ||
| | legal_UN = P I | |||
| | legal_US = Schedule I | | legal_US = Schedule I | ||
| | legal_DE = Anlage I | |||
| | legal_status = | |||
| | addiction_liability = None<ref name="NHM-MDMA"/> | |||
| | routes_of_administration = ], ], ]}} | |||
| | legal_status = ] | |||
| '''Lysergic acid diethylamide''', commonly called '''LSD''', '''LSD-25''', or '''acid''', is a ] ], ] from ] derived from ], a grain fungus that typically grows on rye. The short form LSD comes from the German '''LSDM''', "'''Lyserg säure-diethylamid'''". | |||
| | dependency_liability = Low<ref>{{cite book| vauthors = Halpern JH, Suzuki J, Huertas PE, Passie T | veditors = Price LH, Stolerman IP |title=Encyclopedia of Psychopharmacology A Springer Live Reference|date=June 7, 2014|publisher=Springer-Verlag Berlin Heidelberg|location=Heidelberg, Germany|isbn=978-3-642-27772-6|pages=1–5|quote=Hallucinogen abuse and dependence are known complications resulting from ... LSD and psilocybin. Users do not experience withdrawal symptoms, but the general criteria for substance abuse and dependence otherwise apply. Dependence is estimated in approximately 2 % of recent-onset users |doi=10.1007/978-3-642-27772-6_43-2|chapter=Hallucinogen Abuse and Dependence}}</ref> | |||
| | routes_of_administration = ], ] | |||
| | pronounce = {{IPA|/daɪ eθəl ˈæmaɪd/}}, {{IPA|/æmɪd/}}, or {{IPA|/eɪmaɪd/}}<ref>{{cite encyclopedia |url=http://www.collinsdictionary.com/dictionary/english/amide |title=Definition of "amide" |dictionary=Collins English Dictionary |access-date=January 31, 2015 |url-status=live |archive-url=https://web.archive.org/web/20150402115318/http://www.collinsdictionary.com/dictionary/english/amide |archive-date=April 2, 2015}}</ref><ref>{{cite web |url=https://www.ahdictionary.com/word/search.html?q=amide |title=American Heritage Dictionary Entry: amide |publisher=Ahdictionary.com |access-date=January 31, 2015 |archive-url=https://web.archive.org/web/20150402134025/https://www.ahdictionary.com/word/search.html?q=amide |archive-date=April 2, 2015}}</ref><ref>{{cite web |url=http://www.oxforddictionaries.com/us/definition/english/amide |title=amide – definition of amide in English from the ''Oxford Dictionary''|publisher=Oxforddictionaries.com |access-date=January 31, 2015 |archive-url=https://web.archive.org/web/20150402184403/http://www.oxforddictionaries.com/us/definition/english/amide |archive-date=April 2, 2015}}</ref> | |||
| <!--Pharmacokinetic data-->| bioavailability = 71%<ref name=Dol2015 /> | |||
| LSD is sensitive to ], ], and ], especially in ] (though its potency may last years if it is stored away from light and moisture at low temperature). In pure form it is colorless, odorless and mildly bitter. LSD is typically delivered orally, usually on a substrate such as absorbent blotter paper, a ], or gelatin. In its liquid form, it can be administered by intramuscular or intravenous injection, or even in the form of eye-drops. In its blotter paper form, it is popularly taken by placing a "tab" in the user's mouth for several minutes. The threshold dosage level for an effect on humans is of the order of 20 to 30 micrograms. | |||
| | protein_bound = Unknown<ref name=Pas2008 /> | |||
| | metabolism = ] (])<ref name=Dol2015 /> | |||
| | metabolites = 2-Oxo-3-hydroxy-LSD<ref name=Dol2015 /> | |||
| | onset = 30–40 minutes<ref>{{cite book|vauthors=Neinstein LS|title=Adolescent Health Care: A Practical Guide |date=2008|publisher=Lippincott Williams & Wilkins|isbn=9780781792561|pages=931|url=https://books.google.com/books?id=er8dQPxgcz0C&pg=PA931|language=en|access-date=January 27, 2017|archive-date=December 26, 2018|archive-url=https://web.archive.org/web/20181226025533/https://books.google.com/books?id=er8dQPxgcz0C&pg=PA931|url-status=live}}</ref> | |||
| | duration_of_action = 8–20 hours<ref>{{cite book|vauthors=Kranzler HR, Ciraulo DA|title=Clinical Manual of Addiction Psychopharmacology|publisher=American Psychiatric Pub|isbn=9781585626632|pages=216|url=https://books.google.com/books?id=TYddW0uzIRsC&pg=PA216|language=en|date=2 April 2007|access-date=January 27, 2017|archive-date=December 26, 2018|archive-url=https://web.archive.org/web/20181226025532/https://books.google.com/books?id=TYddW0uzIRsC&pg=PA216|url-status=live}}</ref> | |||
| | elimination_half-life = 3.6 hours<ref name=Dol2015/><ref name=Muc2016/> | |||
| | excretion = ]<ref name=Dol2015 /><ref name=Muc2016 /> | |||
| <!--Identifiers-->| CAS_number_Ref = {{cascite|correct|??}} | |||
| Introduced by ] as a drug with various ] uses, LSD quickly became a ] that appeared to show great promise. However, the extra-medical use of the drug in ] in the middle years of the ] led to a political firestorm that resulted in the ] for medical as well as recreational and spiritual uses. Despite this, it is still considered a promising drug in some intellectual circles, and organizations such as ], ] and the ] exist to fund, encourage and coordinate research into its medical uses. | |||
| | CAS_number = 50-37-3 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 6605 | |||
| | PubChem = 5761 | |||
| | IUPHAR_ligand = 17 | |||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | DrugBank = DB04829 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChemSpiderID = 5558 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 8NA5SWF92O | |||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | KEGG = C07542 | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | ChEMBL = 263881 | |||
| | ATC_prefix = None | |||
| | PDB_ligand = 7LD | |||
| | class = ] (]) | |||
| <!--Chemical data-->| C = 20 | |||
| | H = 25 | |||
| | N = 3 | |||
| | O = 1 | |||
| | smiles = CCN(CC)C(=O)1CN(2Cc3cc4c3c(ccc4)C2=C1)C | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI = 1S/C20H25N3O/c1-4-23(5-2)20(24)14-9-16-15-7-6-8-17-19(15)13(11-21-17)10-18(16)22(3)12-14/h6-9,11,14,18,21H,4-5,10,12H2,1-3H3/t14-,18-/m1/s1 | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey = VAYOSLLFUXYJDT-RDTXWAMCSA-N | |||
| | synonyms = LSD, LSD-25, LAD, acid, lucy, among others | |||
| | melting_point = 80 | |||
| | melting_high = 85 | |||
| | solubility = 67.02<ref>{{cite web|title=Lysergide|url=https://pubchem.ncbi.nlm.nih.gov/compound/5761#section=Solubility|website=pubchem.ncbi.nlm.nih.gov|language=en|access-date=April 12, 2023|archive-date=April 12, 2023|archive-url=https://web.archive.org/web/20230412075752/https://pubchem.ncbi.nlm.nih.gov/compound/5761#section=Solubility|url-status=live}}</ref> | |||
| }} | |||
| '''Lysergic acid diethylamide''', commonly known as '''LSD''' (from German {{lang|de|Lysergsäure-diethylamid}}), is a potent ] that intensifies thoughts, emotions, and sensory perception.<ref name="pmid26841800">{{cite journal |vauthors=Nichols DE |title=Psychedelics |journal=Pharmacological Reviews |volume=68 |issue=2 |pages=264–355 |date=April 2016 |pmid=26841800 |pmc=4813425 |doi=10.1124/pr.115.011478 |veditors=Barker EL |issn=0031-6997}}</ref> Often referred to as '''acid''' or '''lucy''', LSD can cause mystical, spiritual, or religious experiences.<ref>{{cite journal | vauthors = Liechti ME, Dolder PC, Schmid Y | title = Alterations of consciousness and mystical-type experiences after acute LSD in humans | journal = Psychopharmacology | volume = 234 | issue = 9–10 | pages = 1499–1510 | date = May 2017 | pmid = 27714429 | pmc = 5420386 | doi = 10.1007/s00213-016-4453-0 }}</ref><ref>{{cite journal | vauthors = Griffiths RR, Hurwitz ES, Davis AK, Johnson MW, Jesse R | title = Survey of subjective "God encounter experiences": Comparisons among naturally occurring experiences and those occasioned by the classic psychedelics psilocybin, LSD, ayahuasca, or DMT | journal = PLOS ONE | volume = 14 | issue = 4 | pages = e0214377 | date = 2019-04-23 | pmid = 31013281 | pmc = 6478303 | doi = 10.1371/journal.pone.0214377 | doi-access = free | bibcode = 2019PLoSO..1414377G }}</ref> At higher doses, it primarily induces visual and auditory hallucinations.<ref name="LeptourgosFortier-Davy2020">{{cite journal |vauthors=Leptourgos P, Fortier-Davy M, Carhart-Harris R, Corlett PR, Dupuis D, Halberstadt AL, Kometer M, Kozakova E, LarØi F, Noorani TN, Preller KH, Waters F, Zaytseva Y, Jardri R |title=Hallucinations Under Psychedelics and in the Schizophrenia Spectrum: An Interdisciplinary and Multiscale Comparison |journal = Schizophrenia Bulletin |volume=46 |issue=6 |pages=1396–1408 |date=December 2020 |pmid=32944778 |pmc=7707069 |doi=10.1093/schbul/sbaa117 }}</ref><ref name=":3">{{cite journal |vauthors=Holze F, Vizeli P, Ley L, Müller F, Dolder P, Stocker M, Duthaler U, Varghese N, Eckert A, Borgwardt S, Liechti ME |title=Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects |journal=Neuropsychopharmacology |volume=46 |issue=3 |pages=537–544 |date=February 2021 |pmid=33059356 |pmc=8027607 |doi=10.1038/s41386-020-00883-6}}</ref> LSD is not considered addictive, because it does not produce compulsive drug-seeking behavior.<ref>{{Cite web |title=LSD Fast Facts |url=https://www.justice.gov/archive/ndic/pubs4/4260/index.htm |access-date=2024-12-29 |website=www.justice.gov}}</ref> Using LSD can lead to adverse psychological reactions, such as anxiety, paranoia, and delusions.<ref name="Pas2008">{{cite journal |vauthors = Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A |title=The pharmacology of lysergic acid diethylamide: a review |journal=CNS Neuroscience & Therapeutics |volume=14 |issue=4 |pages=295–314 |year=2008 |pmid=19040555 |pmc=6494066 |doi=10.1111/j.1755-5949.2008.00059.x}}</ref> Additionally, it may trigger "flashbacks," also known as ] (HPPD), where individuals experience persistent visual distortions after use.<ref name="NIH2018C"/><ref name="Halpern2018">{{cite book |vauthors=Halpern JH, Lerner AG, Passie T |title=A Review of Hallucinogen Persisting Perception Disorder (HPPD) and an Exploratory Study of Subjects Claiming Symptoms of HPPD. |series=Current Topics in Behavioral Neurosciences |date=2018 |volume=36 |pages=333–360 |isbn=978-3-662-55878-2 |doi=10.1007/7854_2016_457 |pmid=27822679}}</ref> | |||
| The effects of LSD begin within 30 minutes of ingestion and can last up to 20 hours, with most trips averaging 8–12 hours.<ref name="EU2018">{{cite web |title=LSD profile (chemistry, effects, other names, synthesis, mode of use, pharmacology, medical use, control status) |url=http://www.emcdda.europa.eu/publications/drug-profiles/lsd |website=EMCDDA |access-date=14 July 2018 |language=en |archive-date=April 28, 2021 |archive-url=https://web.archive.org/web/20210428113546/https://www.emcdda.europa.eu/publications/drug-profiles/lsd |url-status=live }}</ref><ref name=":1">{{Cite news |vauthors= Sloat S |date= 27 January 2017 |title= This is Why You Can't Escape an Hours-Long Acid Trip |work= Inverse |url= https://www.inverse.com/article/27067-lsd-acid-trip-brain-receptor-serotonin |url-status= live |access-date= 3 February 2020 |archive-date= June 11, 2021 |archive-url= https://web.archive.org/web/20210611212737/https://www.inverse.com/article/27067-lsd-acid-trip-brain-receptor-serotonin }}</ref> It is synthesized from ] and commonly administered via tabs of blotter paper.<ref name="NIH2016">{{cite web |title=What are hallucinogens? |date=January 2016 |url=https://www.drugabuse.gov/publications/drugfacts/hallucinogens |website=National Institute of Drug Abuse |access-date=April 24, 2016 |url-status=live |archive-url=https://web.archive.org/web/20160417180046/https://www.drugabuse.gov/publications/drugfacts/hallucinogens |archive-date=April 17, 2016}}</ref> LSD is mainly used recreationally or for spiritual purposes.<ref name="EU2018" /><ref>{{cite news |vauthors=Gershon L |date=19 July 2016 |title=How LSD Went From Research to Religion |url=https://daily.jstor.org/how-lsd-went-from-research-to-religion/ |access-date=14 July 2018 |work=JSTOR Daily |archive-date=January 28, 2021 |archive-url=https://web.archive.org/web/20210128015545/https://daily.jstor.org/how-lsd-went-from-research-to-religion/ |url-status=live }}</ref> As a serotonin receptor ], LSD's precise effects are not fully understood, but it is known to alter the brain’s ], leading to its powerful psychedelic effects.<ref name="pmid26841800" /><ref name="pmid14761703">{{cite journal |vauthors=Nichols DE |title=Hallucinogens |journal=Pharmacology & Therapeutics |volume=101 |issue=2 |pages=131–181 |date=February 2004 |pmid=14761703 |doi=10.1016/j.pharmthera.2003.11.002 |issn=1879-016X}}</ref><ref>{{Cite journal |vauthors=Girn M, Roseman L, Bernhardt B, Smallwood J, Carhart-Harris R, Spreng RN |date=2020-05-03|title=Serotonergic psychedelic drugs LSD and psilocybin reduce the hierarchical differentiation of unimodal and transmodal cortex |journal=bioRxiv |s2cid=233346402 |doi=10.1101/2020.05.01.072314 |doi-access=free}}</ref> | |||
| The drug was first synthesized by Swiss chemist ] in 1938 and became widely studied in the 1950s and 1960s.<ref name="EU2018" /><ref name="NIH2018C">{{cite web |title=Commonly Abused Drugs Charts |url=https://www.drugabuse.gov/drugs-abuse/commonly-abused-drugs-charts#lsd |website=National Institute on Drug Abuse |access-date=14 July 2018 |date=2 July 2018 |url-status=live |archive-url=https://web.archive.org/web/20200301183029/https://www.drugabuse.gov/drugs-abuse/commonly-abused-drugs-charts#lsd |archive-date=March 1, 2020}}</ref> It was used experimentally in ] for treating ] and ].<ref name="Use of d-lysergic acid diethylamide">{{cite journal |vauthors=Chwelos N, Blewett DB, Smith CM, Hoffer A |title=Use of d-lysergic acid diethylamide in the treatment of alcoholism |journal=Quarterly Journal of Studies on Alcohol |volume=20 |issue=3 |pages=577–590 |date=September 1959 |pmid=13810249 |doi=10.15288/qjsa.1959.20.577}}</ref> However, its association with the ] of the 1960s led to its classification as a ] drug in the U.S. in 1968.<ref>{{Cite book |author=United States Congress House Committee on Interstate and Foreign Commerce Subcommittee on Public Health and Welfare |url=https://books.google.com/books?id=qbY6xQEACAAJ |title=Increased Controls Over Hallucinogens and Other Dangerous Drugs |date=1968 |publisher=U.S. Government Printing Office |access-date=August 3, 2021|archive-date=July 13, 2020 |archive-url=https://web.archive.org/web/20200713014802/https://books.google.com/books?id=qbY6xQEACAAJ|url-status=live}}</ref> It was also listed as a ] by the ] in 1971 and remains without approved medical uses.<ref name="EU2018" /> | |||
| Despite its legal restrictions, LSD remains influential in scientific and cultural contexts. Its therapeutic potential has been explored, particularly in treating mental health disorders.<ref name="pmid26841800" /><ref>{{Cite web |title=Psychiatric Research with Hallucinogens |website=www.druglibrary.org |url=https://www.druglibrary.org/schaffer/lsd/grob.htm |access-date=2021-07-26|archive-date=July 26, 2021|archive-url=https://web.archive.org/web/20210726203733/https://www.druglibrary.org/schaffer/lsd/grob.htm |url-status=live}}</ref> As of 2017, about 10% of people in the U.S. had used LSD at some point, with 0.7% having used it in the past year.<ref name="NIH2018B">{{cite web|author=National Institute on Drug Abuse|title=Hallucinogens |url=https://www.drugabuse.gov/drugs-abuse/hallucinogens |access-date=14 July 2018|archive-date=June 3, 2020|archive-url=https://web.archive.org/web/20200603125635/https://www.drugabuse.gov/drugs-abuse/hallucinogens|url-status=live}}</ref> Usage rates have risen, with a 56.4% increase in adult use in the U.S. from 2015 to 2018.<ref>{{cite journal |vauthors=Yockey RA, Vidourek RA, King KA |title=Trends in LSD use among US adults: 2015–2018 |journal=Drug and Alcohol Dependence |volume=212 |pages=108071 |date=July 2020 |pmid=32450479 |doi=10.1016/j.drugalcdep.2020.108071 |s2cid=218893155}}</ref> | |||
| ==Origins and history== | |||
| {{TOC limit}} | |||
| ] | |||
| {{main|History of LSD}} | |||
| LSD was first synthesized on ], ] by Swiss chemist Dr. ] at the ] in ], ], as part of a large research program searching for medically useful ] derivatives. Its ] properties were unknown until 5 years later, when Hofmann, acting on what he has called a "peculiar presentiment," returned to work on the chemical. He attributed the discovery of the compound's psychoactive effects to the accidental absorption of a tiny amount through his skin on ], which led to him testing a larger amount (250 µg) on himself for ] on April 19.<ref name="problem-child">Hofmann, Albert. ''LSD—My Problem Child'' (McGraw-Hill, 1980). ISBN 0-07-029325-2. Available online or ; accessed ].</ref> | |||
| ==Uses== | |||
| Until 1966, LSD and ] were provided by Sandoz Laboratories free of charge to interested scientists under the ] "Delysid".<ref name="problem-child"/> The use of these compounds by ]s to gain a better subjective understanding of the ] experience was an accepted practice. Many ] were conducted on the potential use of LSD in ], generally with very positive results. | |||
| ===Recreational=== | |||
| ==Regulation and research== | |||
| LSD is commonly used as a recreational drug.<ref>{{cite web|title=DrugFacts: Hallucinogens – LSD, Peyote, Psilocybin, and PCP |url=http://www.drugabuse.gov/publications/drugfacts/hallucinogens-lsd-peyote-psilocybin-pcp|publisher=National Institute on Drug Abuse |access-date=February 17, 2015|date=December 2014|archive-url=https://web.archive.org/web/20150216030833/http://www.drugabuse.gov/publications/drugfacts/hallucinogens-lsd-peyote-psilocybin-pcp |archive-date=February 16, 2015}}</ref> | |||
| ===Spiritual=== | |||
| ] intelligence services were keenly interested in the possibilities of using LSD for ] and ], and also for large-scale ]. The ] conducted extensive research on LSD, which was mostly destroyed.<ref>ACHRE Report, chapter 3: "".</ref> LSD was a central research area for ], the code name for a CIA mind-control research program begun in the 1950s and continued until the late 1960s. Tests were also conducted by the ] (now known as the U.S. Army ]) located in the ] at ]. Volunteers would take LSD and then perform a battery of tests to investigate the effects of the drug on ]s. Based on remaining publicly available records, the projects seem to have concluded that LSD was of little practical use as a mind control drug and moved on to other drugs. Both the CIA and the Army experiments became highly controversial when they became public knowledge in the ], as the test subjects were not normally informed of the nature of the experiments, or even that they were subjects in experiments at all. Several subjects developed severe ] and even committed ] after the experiments. The controversy contributed to ]'s creation of the ] and new regulations on ]. | |||
| LSD can catalyze intense spiritual experiences and is thus considered an ]. Some users have reported ] experiences. In 1966, ] established the ] with LSD as its ].<ref>{{cite book | title = Alcohol and Drugs in North America: A Historical Encyclopedia | veditors = Fahey D, Miller JS | isbn = 978-1-59884-478-8 | page = 375 }}</ref><ref>'']'' September 20, 1966 Page One</ref> ] has written that religious and mystical experiences observed during LSD sessions appear to be ] indistinguishable from similar descriptions in the ] of the great religions of the world and the texts of ancient ]s.<ref name="Grof1979">{{cite book| vauthors=Grof S, Grof JH| author-link1=Stanislav Grof| title=Realms of the Human Unconscious (Observations from LSD Research)| publisher=Souvenir Press (E & A) Ltd| year=1979| location=London| pages=13–14| url=http://www.csp.org/chrestomathy/realms_of3.html| isbn=978-0-285-64882-1| archive-url=https://web.archive.org/web/20071018164416/http://csp.org/chrestomathy/realms_of3.html| archive-date=October 18, 2007| access-date=November 18, 2007}}</ref> | |||
| ===Medical=== | |||
| The ] government also engaged in LSD testing; in 1953 and 1954, scientists working for ] dosed servicemen in an effort to find a "truth drug". The test subjects were not informed that they were being given LSD, and had in fact been told that they were participating in a medical project to find a cure for the ]. One subject, aged 19 at the time, reported seeing "walls melting, cracks appearing in people's faces … eyes would run down cheeks, ]-type faces … a flower would turn into a slug". After keeping the trials secret for many years, MI6 agreed in 2006 to pay the former test subjects financial compensation. Like the CIA, MI6 decided that LSD was not a practical drug for mind control purposes.<ref>Rob Evans, "". ''The Guardian'' 24 February 2006.</ref> | |||
| {{See also|Lysergic acid diethylamide#Research}} | |||
| LSD currently has no approved uses in ].<ref name=Nutt2009>{{cite journal |vauthors=Nutt DJ, King LA, Nichols DE |title=Effects of Schedule I drug laws on neuroscience research and treatment innovation |journal=Nature Reviews. Neuroscience |volume=14 |issue=8 |pages=577–585 |date=August 2013 |pmid=23756634 |doi=10.1038/nrn3530 |s2cid=1956833}}</ref><ref>{{Cite news |url=https://www.theguardian.com/science/2009/oct/23/lsd-ecstacy-health-benefits |title=Scientists study possible health benefits of LSD and ecstasy {{!}} Science |date=2016-07-23 |access-date=2016-07-23 |url-status= live |archive-url=https://web.archive.org/web/20160723155424/https://www.theguardian.com/science/2009/oct/23/lsd-ecstacy-health-benefits |archive-date=July 23, 2016 |newspaper=The Guardian |vauthors=Campbell D}}</ref> A ] concluded that a single dose was shown to be effective at reducing alcohol consumption in people suffering from ].<ref name="Lysergic acid diethylamide LSD fo"/> LSD has also been studied in ], ],<ref name=":5">{{Cite news |vauthors=Lustberg D |date=2022-10-14 |title=Acid for Anxiety: Fast and Lasting Anxiolytic Effects of LSD |url=https://psychedelicreview.com/acid-for-anxiety-fast-and-lasting-anxiolytic-effects-of-lsd/ |access-date=2022-12-01 |website=Psychedelic Science Review |language=en-US |archive-date=December 1, 2022 |archive-url=https://web.archive.org/web/20221201092629/https://psychedelicreview.com/acid-for-anxiety-fast-and-lasting-anxiolytic-effects-of-lsd/ |url-status=live }}</ref><ref name=":6">{{cite journal |vauthors=Holze F, Gasser P, Müller F, Dolder PC, Liechti ME |title=Lysergic Acid Diethylamide-Assisted Therapy in Patients With Anxiety With and Without a Life-Threatening Illness: A Randomized, Double-Blind, Placebo-Controlled Phase II Study |journal=Biological Psychiatry |date=September 2022 |volume=93 |issue=3 |pages=215–223 |doi=10.1016/j.biopsych.2022.08.025 |pmid=36266118 |s2cid=252095586 |doi-access=free}}</ref> and ], with positive preliminary results.<ref>{{cite journal |vauthors=Dos Santos RG, Osório FL, Crippa JA, Riba J, Zuardi AW, Hallak JE |title=Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years |journal=Therapeutic Advances in Psychopharmacology |volume=6 |issue=3 |pages=193–213 |date=June 2016 |pmid=27354908 |pmc=4910400 |doi=10.1177/2045125316638008}}</ref><ref>{{Cite web |title=History of LSD Therapy |url=https://druglibrary.org/schaffer/lsd/grofhist.htm |access-date=2022-11-07 |website=druglibrary.org |archive-date=November 7, 2022 |archive-url=https://web.archive.org/web/20221107004840/https://druglibrary.org/schaffer/lsd/grofhist.htm |url-status=live }}</ref> | |||
| ==Effects== | |||
| LSD first became popular ] among a small group of mental health professionals such as psychiatrists and psychologists during the 1950s, as well as by socially prominent and politically powerful individuals such as ] and ] to whom the early LSD researchers were connected socially. | |||
| LSD is exceptionally potent, with as little as 20 μg capable of producing a noticeable effect.<ref name="EU2018" /> | |||
| ===Physical=== | |||
| Several mental health professionals involved in LSD research, most notably ] psychology professors Dr. ] and ], became convinced of LSD's potential as a tool for ] growth. In 1961, Dr. Timothy Leary received grant money from Harvard University to study the effects of LSD on test subjects. 3,500 doses were given to over 400 people. Of those tested, 90% said they would like to repeat the experience, 83% said they had "learned something or had insight," and 62% said it had changed their life for the better. | |||
| ] | |||
| ] (pupil dilation) due to usage of LSD]] | |||
| LSD can induce physical effects such as ], decreased ], increased ], and ]. The physical reactions to LSD vary greatly and some may be a result of its psychological effects. Commonly observed symptoms include increased ], ], and ], as well as ], ], ], and ]. In cases of adverse reactions, users may experience ], ], ], and ].<ref name="EU2018" /> | |||
| ===Psychological=== | |||
| Their research became more ] and controversial, as Leary and Alpert alleged links between the LSD experience and the state of ] sought after in many ] traditions. They were dismissed from the traditional academic psychology community, and as such cut off from legal scientific acquisition of the drug. Drs. Leary and Alpert somehow acquired a quantity of LSD and relocated to a private mansion, where they continued their research. The experiments lost their scientific character as the pair evolved into ] ] ]s associated with the ] movement, encouraging people to question authority and challenge the status quo, a concept summarized in Leary's catchphrase, "]". | |||
| The primary immediate psychological effects of LSD are ]s and altered thought, often referred to as "trips". These sensory alterations are considered pseudohallucinations because the subject does not perceive the patterns seen as being located in three-dimensional space outside the body.<ref>{{cite journal | vauthors = El-Mallakh RS, Walker KL | title = Hallucinations, psuedohallucinations, and parahallucinations | journal = Psychiatry | volume = 73 | issue = 1 | pages = 34–42 | date = 2010 | pmid = 20235616 | doi = 10.1521/psyc.2010.73.1.34 }}</ref> LSD is not considered addictive.<ref>{{Cite web |title=LSD Fast Facts |url=https://www.justice.gov/archive/ndic/pubs4/4260/index.htm |access-date=2024-12-29 |website=www.justice.gov}}</ref> These effects typically begin within 20–30 minutes of oral ingestion, peak three to four hours after ingestion, and can last up to 20 hours, particularly with higher doses. An "]" effect, characterized by an improved mood or perceived mental state, may persist for days or weeks following ingestion.<ref>{{cite journal |vauthors=Majić T, Schmidt TT, Gallinat J |title=Peak experiences and the afterglow phenomenon: when and how do therapeutic effects of hallucinogens depend on psychedelic experiences? |journal=Journal of Psychopharmacology |volume=29 |issue=3 |pages=241–253 |date=March 2015 |pmid=25670401 |doi=10.1177/0269881114568040 |s2cid=16483172}}</ref> Positive experiences, or "good trips", are described as intensely pleasurable and can include feelings of joy, euphoria, an increased appreciation for life, decreased anxiety, a sense of spiritual enlightenment, and a feeling of interconnectedness with the universe.<ref name="erowid-faq">{{cite web |work=] |vauthors=Honig D |title=Frequently Asked Questions |url=http://www.erowid.org/chemicals/lsd/lsd_faq.shtml |archive-url=https://web.archive.org/web/20160212232436/https://www.erowid.org/chemicals/lsd/lsd_faq.shtml |archive-date=12 February 2016}}</ref><ref name="PMID6054248">{{cite journal |vauthors=McGlothlin W, Cohen S, McGlothlin MS |title=Long lasting effects of LSD on normals |journal=Archives of General Psychiatry |volume=17 |issue=5 |pages=521–532 |date=November 1967 |pmid=6054248 |doi=10.1001/archpsyc.1967.01730290009002| url=http://www.maps.org/w3pb/new/1967/1967_mcglothlin_4655_1.pdf |archive-date=April 30, 2011 |archive-url=https://web.archive.org/web/20110430020912/http://www.maps.org/w3pb/new/1967/1967_mcglothlin_4655_1.pdf}}</ref> | |||
| Negative experiences, commonly known as "]s", can induce feelings of fear, agitation, anxiety, panic, and paranoia.<ref name="Pas2008"/><ref name="kopra_adverse">{{cite journal | vauthors = Kopra EI, Ferris JA, Rucker JJ, McClure B, Young AH, Copeland CS, Winstock AR | title = Adverse experiences resulting in emergency medical treatment seeking following the use of lysergic acid diethylamide (LSD) | journal = Journal of Psychopharmacology | volume = 36 | issue = 8 | pages = 956–964 | date = August 2022 | pmid = 35672900 | pmc = 9353972 | doi = 10.1177/02698811221099650 }}</ref> While the occurrence of a bad trip is unpredictable, factors such as mood, surroundings, sleep, hydration, and social setting, collectively referred to as "]", can influence the risk and are considered important in minimizing the likelihood of a negative experience.<ref name=MedlinePlus>{{citation |title=Substance use – LSD |vauthors=Rogge T |date=21 May 2014 |access-date=14 July 2016 |publisher=MedlinePlus, U.S. National Library of Medicine |url=https://medlineplus.gov/ency/patientinstructions/000795.htm |url-status=live|archive-url=https://web.archive.org/web/20160728004220/https://medlineplus.gov/ency/patientinstructions/000795.htm|archive-date=July 28, 2016}}</ref><ref name=CESAR>{{citation|title=LSD |author=CESAR |publisher=Center for Substance Abuse Research, University of Maryland |date=29 October 2013 |access-date=14 July 2016 |url=http://www.cesar.umd.edu/cesar/drugs/lsd.asp |archive-url=https://web.archive.org/web/20160715071823/http://www.cesar.umd.edu/cesar/drugs/lsd.asp |archive-date=July 15, 2016}}</ref> | |||
| The drug was banned in the United States in 1967, with scientific therapeutic research as well as individual research also becoming prohibitively difficult. Many other countries, under pressure from the U.S., quickly followed suit. Since 1967, underground recreational and therapeutic LSD use has continued in many countries, supported by a black market and popular demand for the drug. Legal, academic research experiments on the effects and mechanisms of LSD are also conducted on occasion, but rarely involve human subjects. Despite its proscription, the ] counterculture continued to promote the regular use of LSD, led by figures such as Leary and ] bands such as ] and ]. | |||
| ===Sensory=== | |||
| ] has been used as a term (often derogatory) for one who frequently uses LSD. | |||
| LSD induces an animated sensory experience affecting senses, emotions, memories, time, and awareness, lasting from 6 to 20 hours, with the duration dependent on dosage and individual tolerance. Effects typically commence within 30 to 90 minutes post-ingestion, ranging from subtle perceptual changes to profound ]s. Alterations in auditory and visual perception are common.<ref name="linton-langs"/><ref>{{cite journal |vauthors=Katz MM, Waskow IE, Olsson J |title=Characterizing the psychological state produced by LSD |journal=Journal of Abnormal Psychology |volume=73 |issue=1 |pages=1–14 |date=February 1968 |pmid=5639999 |doi=10.1037/h0020114 |citeseerx=10.1.1.409.4030}}</ref> | |||
| Users may experience enhanced visual phenomena, such as vibrant colors, objects appearing to morph, ripple or move, and geometric patterns on various surfaces. Changes in the perception of food's texture and taste are also noted, sometimes leading to aversion towards certain foods.<ref name="linton-langs">{{cite journal |journal=Archives of General Psychiatry |title=Subjective Reactions to Lysergic Acid Diethylamide (LSD-25) |date=May 1962 |volume=6 |issue=5 |pages=352–368 |doi=10.1001/archpsyc.1962.01710230020003 |vauthors=Linton HR, Langs RJ}}</ref><ref>{{cite journal |vauthors=Parker LA |title=LSD produces place preference and flavor avoidance but does not produce flavor aversion in rats |journal=Behavioral Neuroscience |volume=110 |issue=3 | pages=503–508 |date=June 1996 |pmid=8888996 |doi=10.1037/0735-7044.110.3.503}}</ref> | |||
| According to ] and ], two researchers associated with the ] who performed a 1994 review of the literature, LSD use is relatively uncommon when compared to the abuse of ], ], and ]. Over the previous fifteen years, long-term usage trends stayed fairly stable, with roughly 5% of the population using the drug and most users being in the 16 to 23 age range.<ref>{{cite journal | |||
| | url = http://www.maps.org/news-letters/v06n1/06154lsd.html | |||
| | title = A Review of "LSD : Still With Us After All These Years" | |||
| | journal = Newsletter of the Multidisciplinary Association for Psychedelic Studies | |||
| | first = Neal M. | |||
| | last = Goldsmith | |||
| | year = 1995 | |||
| | volume = 6 | |||
| | issue = 1 | |||
| | accessdate = 2006-01-31}}</ref> Henderson and Glass found that LSD users typically partook of the substance on an infrequent, episodic basis, then "maturing out" after two to four years. Overall, LSD appeared to have comparatively few adverse health consequences, of which "]s" were the most commonly reported (and, the researchers found, one of the chief reasons youths stop using the drug).<ref name="henderson-glass">{{cite book|author= Henderson, Leigh A.; Glass, William J.|title=LSD: Still with Us after All These Years|year=1994|Jossey-Bass Inc., San Francisco|id = ISBN 978-0787943790}}</ref> | |||
| There are reports of inanimate objects appearing animated, with static objects seeming to move in additional spatial dimensions.<ref>{{cite journal |vauthors=Oster G |title=Moiré patterns and visual hallucinations |journal=Psychedelic Review |date=1966 |volume=7 |pages=33–40 |url= https://maps.org/research-archive/psychedelicreview/n07/n07033osl.pdf |url-status=live |archive-date=19 April 2017 |archive-url=https://web.archive.org/web/20170419154504/http://www.maps.org/research-archive/psychedelicreview/n07/n07033osl.pdf}}</ref> The auditory effects of LSD may include ]-like distortions of sounds, and an intensified experience of music. Basic visual effects often resemble ] and can be influenced by concentration, thoughts, emotions, or music.<ref>{{cite journal |vauthors=Kaelen M, Roseman L, Kahan J, Santos-Ribeiro A, Orban C, Lorenz R, Barrett FS, Bolstridge M, Williams T, Williams L, Wall MB, Feilding A, Muthukumaraswamy S, Nutt DJ, Carhart-Harris R |date=July 2016 |title=LSD modulates music-induced imagery via changes in parahippocampal connectivity |journal=European Neuropsychopharmacology |volume=26 |issue=7 |pages=1099–1109 |pmid=27084302 |doi=10.1016/j.euroneuro.2016.03.018 |s2cid=24037275}}</ref> Higher doses can lead to more intense sensory perception alterations, including synesthesia, perception of additional dimensions, and temporary ]. | |||
| == Dosage == | |||
| LSD is, by mass, one of the most potent drugs yet discovered. Dosages of LSD are measured in ]s (µg), or millionths of a ]. By comparison, dosages of almost all other drugs, both recreational and medical, are measured in ]s (mg), or thousandths of a gram. ] determined that an active dose of ], roughly 0.2 to 0.5 g, has effects comparable to 100 µg or less of LSD; put another way, LSD is between five and ten thousand times more active than mescaline.<ref name="problem-child"/> | |||
| ==Adverse effects== | |||
| While a typical single dose of LSD may be between 100 and 500 micrograms — an amount roughly equal to one-tenth the mass of a grain of sand — threshold effects can be felt with as little as 20 micrograms.<ref name="greiner">{{cite journal | author=Greiner T, Burch NR, Edelberg R | title=Psychopathology and psychophysiology of minimal LSD-25 dosage; a preliminary dosage-response spectrum | journal=AMA Arch Neurol Psychiatry | year=1958 | pages=208–10 | volume=79 | issue=2 | id=PMID 13497365}}</ref> | |||
| ] | |||
| ] regarding 20 popular recreational drugs. LSD was ranked 14th in dependence, 15th in physical harm, and 13th in social harm.<ref>{{cite journal |vauthors=Nutt D, King LA, Saulsbury W, Blakemore C |title=Development of a rational scale to assess the harm of drugs of potential misuse |journal=Lancet |volume=369 |issue=9566 |pages=1047–53 |date=March 2007 |pmid=17382831 |doi=10.1016/s0140-6736(07)60464-4 |s2cid=5903121}}</ref>]] | |||
| LSD, a classical psychedelic, is deemed physiologically safe at standard dosages (50–200 μg) and its primary risks lie in psychological effects rather than physiological harm.<ref name="pmid14761703"/><ref name="pmid29408722">{{cite journal |journal=Forensic Science International |vauthors=Nichols DE, Grob CS |doi=10.1016/j.forsciint.2018.01.006 |title=Is LSD Toxic? |volume=284 |pages=141–145 |date=March 2018 |pmid=29408722}}</ref> A 2010 study by ] ranked LSD as significantly less harmful than ], placing it near the bottom of a list assessing the harm of 20 drugs.<ref name="pmid21036393">{{cite journal |vauthors=Nutt DJ, King LA, Phillips LD |title=Drug harms in the UK: a multicriteria decision analysis |journal=Lancet |volume=376 |issue=9752 |pages=1558–65 |date=November 2010 |pmid=21036393 |doi=10.1016/s0140-6736(10)61462-6 |s2cid=5667719 |citeseerx=10.1.1.690.1283}}</ref> | |||
| According to Stoll, the dosage level that will produce a threshold hallucinogenic effect in humans is generally considered to be 20 to 30 μg, with the drug's effects becoming markedly more evident at higher dosages.<ref>Stoll, W.A. (1947). Ein neues, in sehr kleinen Mengen wirsames Phantastikum. Schweiz. Arch. Neur. 60,483.</ref><ref name="greiner"/> According to Glass and Henderson's review, black-market LSD is largely unadulterated though sometimes contaminated by manufacturing by-products. Typical doses in the 1960s ranged from 200 to 1000 µg, while street samples of the 1970s contained 30 to 300 µg. By the mid-1980s, the average had reduced to about 100 to 125 µg, lowering still further in the 1990s to the 20–80 µg range. (Lower doses, Glass and Henderson found, generally produce fewer ]s.)<ref name="henderson-glass"/> Dosages by frequent users can be as high as 1,200 µg (1.2 mg), although such a high dosage may precipitate unpleasant physical and psychological reactions. | |||
| ===Psychological effects=== | |||
| Estimates for the lethal dosage (]) of LSD range from 200 μg/kg to more than 1 mg/kg of human body mass, though most sources report that there are no known human cases of such an overdose. Other sources note one report of a suspected fatal overdose of LSD occuring in ] in ] in which there were indications that ~1/3 of a gram (320 mg or 320,000 µg) had been injected intravenously, i.e., over 3,000 more typical oral doses of ~100 µg had been injected.<ref name="erowid-dosage">{{cite web | url = http://www.erowid.org/chemicals/lsd/lsd_dose.shtml | |||
| ====Mental disorders==== | |||
| | title = LSD Vault: Dosage | |||
| LSD can induce ] or extreme anxiety, colloquially termed a "]". Despite lower rates of depression and substance abuse found in psychedelic drug users compared to controls, LSD presents heightened risks for individuals with severe mental illnesses like ].<ref>{{cite journal |vauthors=Krebs TS, Johansen PØ |title=Psychedelics and mental health: a population study |journal=PLOS ONE |volume=8 |issue=8 |pages=e63972 |date=2013-08-19 |pmid=23976938 |pmc=3747247 |doi=10.1371/journal.pone.0063972 |bibcode=2013PLoSO...863972K |veditors=Lu L |doi-access=free}}</ref><ref name="Murray">{{citation |title=What can we learn about schizophrenia from studying the human model, drug-induced psychosis?|vauthors=Murray RM, Paparelli A, Morrison PD, Marconi A, Di Forti M |journal=American Journal of Medical Genetics Part B |volume=162 |issue=7 |series=Special Issue: Identifying the Origins of Mental Illness: A Festschrift in Honor of Ming T. Tsuang |pages=661–670 |date=October 2013 |pmid=24132898 |s2cid=205326399 |doi=10.1002/ajmg.b.32177 |doi-access=free}}</ref> These hallucinogens can catalyze psychiatric disorders in predisposed individuals, although they do not tend to induce illness in emotionally healthy people.<ref name="pmid14761703"/> | |||
| | publisher = ] | |||
| | date = ] | |||
| | accessdate = 2007-01-31}}</ref> | |||
| ====Suggestibility==== | |||
| LSD is not considered addictive, in that its users do not exhibit the medical community's commonly accepted definitions of ] and physical dependence. Rapid tolerance build-up prevents regular use, and there is cross-tolerance shown between LSD, ] and ]. This tolerance diminishes after a few days' abstention from use. | |||
| While research from the 1960s indicated increased suggestibility under the influence of LSD among both mentally ill and healthy individuals, recent documents suggest that the CIA and Department of Defense have discontinued research into LSD as a means of mind control.<ref>{{cite web |url= http://www.gulfweb.org/bigdoc/rockrep.cfm#hallucinogens |title=Is Military Research Hazardous to Veterans Health? Lessons Spanning Half A Century, part F. HALLUCINOGENS |publisher=103rd Congress, 2nd Session-S. Prt. 103-97; Staff Report prepared for the committee on veterans' affairs |date=December 8, 1994 |vauthors=Rockefeller IV JD |location=West Virginia |archive-url=https://web.archive.org/web/20060813164326/http://gulfweb.org/bigdoc/rockrep.cfm#hallucinogens |archive-date=August 13, 2006 |access-date=December 13, 2018}}</ref><ref>{{cite journal |vauthors=Middlefell R |title=The effects of LSD on body sway suggestibility in a group of hospital patients |journal=The British Journal of Psychiatry |volume=113 |issue=496 |pages=277–280 |date=March 1967 |pmid=6029626 |doi=10.1192/bjp.113.496.277 |s2cid=19439549 |url= http://www.lycaeum.org/research/researchpdfs/1489.pdf |archive-url=https://web.archive.org/web/20110430033215/http://www.lycaeum.org/research/researchpdfs/1489.pdf |archive-date=2011-04-30}}</ref><ref>{{cite journal |vauthors=Sjoberg BM, Hollister LE |title=The effects of psychotomimetic drugs on primary suggestibility |journal=Psychopharmacologia |volume=8 |issue=4 |pages=251–262 |date=November 1965 |pmid=5885648 |doi=10.1007/BF00407857 |s2cid=15249061}}</ref>{{Primary source inline|date=June 2023}} | |||
| ====Flashbacks==== | |||
| ] are psychological episodes where individuals re-experience some of LSD's subjective effects after the drug has worn off, persisting for days or months post-] use.<ref name="Halpern2003">{{cite journal |vauthors=Halpern JH, Pope HG |title=Hallucinogen persisting perception disorder: what do we know after 50 years? |journal=Drug and Alcohol Dependence |volume=69 |issue=2 |pages=109–19 |date=March 2003 |pmid=12609692 |doi=10.1016/S0376-8716(02)00306-X}}</ref><ref>{{cite journal |vauthors=Müller F, Kraus E, Holze F, Becker A, Ley L, Schmid Y, Vizeli P, Liechti ME, Borgwardt S |title=Flashback phenomena after administration of LSD and psilocybin in controlled studies with healthy participants |journal=Psychopharmacology |date=January 2022 |volume=239 |issue=6 |pages=1933–1943 |pmid=35076721 |doi=10.1007/s00213-022-06066-z |pmc=9166883 |s2cid=246276633}}</ref> These experiences are associated with ] (HPPD), where flashbacks occur intermittently or chronically, causing distress or functional impairment.<ref name="Halpern2018"/> | |||
| The etiology of flashbacks is varied. Some cases are attributed to ], where individuals fixate on normal ] experiences previously unnoticed prior to drug consumption.<ref>{{cite journal |vauthors=Johansen PØ, Krebs TS |title=Psychedelics not linked to mental health problems or suicidal behavior: a population study |journal=Journal of Psychopharmacology |volume=29 |issue=3 |pages=270–279 |date=March 2015 |doi=10.1177/0269881114568039 |pmid=25744618 |s2cid=2025731}}</ref> Other instances are linked to associative reactions to contextual cues, similar to responses observed in individuals with past trauma or emotional experiences.<ref>{{cite book |vauthors=Holland D, Passie T |isbn=978-3-86135-207-5 |language=de |year=2011 |title=Flashback-Phänomene als Nachwirkung von Halluzinogeneinnahme |volume=2 |series=Bewusstsein – Kognition – Erleben |publisher=VWB Report |url=http://www.vwb-verlag.com/Katalog/m207.html |access-date=June 9, 2023 |archive-date=June 9, 2023 |archive-url=https://web.archive.org/web/20230609015208/http://www.vwb-verlag.com/Katalog/m207.html |url-status=live }}</ref> The risk factors for flashbacks remain unclear, but pre-existing psychopathologies may be significant contributors.<ref>{{cite journal |vauthors=Abraham HD, Duffy FH |date=October 1996 |title=Stable quantitative EEG difference in post-LSD visual disorder by split-half analysis: evidence for disinhibition |journal=Psychiatry Research |volume=67 |issue=3 |pages=173–87 |pmid=8912957 |doi=10.1016/0925-4927(96)02833-8 |s2cid=7587687}}</ref> | |||
| IT"S DELICIOUSFUL!!!!!! | |||
| Estimating the prevalence of HPPD is challenging. It is considered rare, with occurrences ranging from 1 in 20 users experiencing the transient and less severe type 1 HPPD, to 1 in 50,000 for the more concerning type 2 HPPD.<ref name="Halpern2018"/> Contrary to internet rumors, LSD is not stored long-term in the ] or other body parts. Pharmacological evidence indicates LSD has a half-life of 175 minutes and is metabolized into water-soluble compounds like 2-oxo-3-hydroxy-LSD, eliminated through urine without evidence of long-term storage.<ref name="Pas2008"/> Clinical evidence also suggests that chronic use of ]s can potentiate LSD-induced flashbacks, even months after stopping LSD use.<ref name="drug-interaction">{{cite book |title=Psychedelics as Psychiatric Medications |publisher=] |isbn=9780192678522 |url=https://books.google.com/books?id=7lazEAAAQBAJ |date=7 March 2023 |vauthors=Nutt DJ, Castle D |chapter=Drug-interaction with psychotropic drugs |access-date=May 21, 2023 |archive-date=May 21, 2023 |archive-url=https://web.archive.org/web/20230521000115/https://books.google.com/books?id=7lazEAAAQBAJ |url-status=live }}</ref>{{rp|145}} | |||
| == Chemistry == | |||
| ] | |||
| ===Drug interactions=== | |||
| LSD is an example of an ] derivative. It is commonly produced from ], which is made from ], a substance derived from the ] ] on ], or from ] (lysergic acid amide). LSA, lysergic acid amide, is the sister chemical of LSD found in ] and ] seeds. It is theoretically possible to manufacture LSD from morning glory or hawaiian baby woodrose seed. LSD is a ] compound with two ]s at the ] atoms C-5 and C-8, so that theoretically four different ] of LSD could exist. LSD, also called (+)-<small>D</small>-LSD, has the ] (5''R'',8''R''). The C-5 ]s of lysergamides do not exist in nature and are not formed during the synthesis from <small>D</small>-lysergic acid. However, LSD and iso-LSD, the two C-8 isomers, rapidly interconvert in the presence of ]. Non-psychoactive iso-LSD which has formed during the synthesis can be removed by ] and can be isomerized to LSD. | |||
| Several psychedelics, including LSD, are metabolized by ]. Concurrent use of ], potent inhibitors of CYP2D6, with LSD may heighten the risk of ].<ref name="drug-interaction"/>{{rp|145}} Chronic usage of SSRIs, ]s, and ]s is believed to diminish the subjective effects of psychedelics, likely due to SSRI-induced 5-HT<sub>2A</sub> receptor downregulation and MAOI-induced 5-HT<sub>2A</sub> receptor desensitization.<ref name="Pas2008"/><ref name="drug-interaction"/>{{rp|145}} Interactions between psychedelics and ]s or ]s are not well-documented; however, co-use with mood stabilizers like ] may induce ]s and ], particularly in individuals with ].<ref name="drug-interaction"/>{{rp|146}}<ref>{{cite journal |journal=Drug and Alcohol Dependence |volume=239 |at=109586 |vauthors=Simonsson O, Goldberg SB, Chambers R, Osika W, Long DM, Hendricks PS |date=1 October 2022 |pmid=35981469 |pmc=9627432 |title=Prevalence and associations of classic psychedelic-related seizures in a population-based sample |doi=10.1016/j.drugalcdep.2022.109586}}</ref><ref>{{cite journal |year=1967 |journal=Western Journal of Medicine |vauthors=Fisher D, Ungerleider J |pmid=4962683 |pmc=1502729 |volume=106 |issue=3 |pages=201–211 |title=Grand mal seizures following ingestion of LSD}}</ref> Lithium notably intensifies LSD reactions, potentially leading to acute comatose states when combined.<ref name="Pas2008" /> | |||
| === |
===Lethal dose=== | ||
| The lethal oral dose of LSD in humans is estimated at 100 mg, based on LD<sub>50</sub> and lethal blood concentrations observed in rodent studies.<ref name="pmid29408722" /> | |||
| "LSD," writes the chemist ], "is an unusually fragile molecule."<ref name="tihkal"/> It is stable for indefinite amounts of time ''if'' stored, as a salt or in water, at low temperature and protected from air and light exposure. Two portions of its molecular structure are particularly sensitive, the carboxamide attachment at the 8-position and the ] between the 8-position and the ]. The former is affected by high ], and if perturbed will produce isolysergic acid diethylamide (iso-LSD), which is biologically inactive. If water or alcohol adds to the double bond (especially in the presence of light), LSD converts to "lumi-LSD", which is totally inactive in human beings, to the best of current knowledge. Furthermore, ] destroys LSD molecules on contact; even though chlorinated tap water typically contains only a slight amount of chlorine, because a typical LSD solution only contains a small amount of LSD, dissolving LSD in tap water is likely to completely eliminate the substance.<ref name="tihkal"/> | |||
| ===Tolerance=== | |||
| A controlled study was undertaken to determine the stability of LSD in pooled urine samples.<ref>{{cite journal | author=Li Z., McNally A. J., Wang H., Salamone S. J. | url=http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9788528&dopt=Abstract | title=Stability study of LSD under various storage conditions. | journal=J Anal Toxicol | volume=22 | issue=6 | pages=520–5 | month=October | year=1998 | id=PMID 9788528}}</ref> The concentrations of LSD in urine samples were followed over time at various temperatures, in different types of storage containers, at various exposures to different wavelengths of light, and at varying pH values. These studies demonstrated no significant loss in LSD concentration at 25 degrees C for up to 4 weeks. After 4 weeks of incubation, a 30% loss in LSD concentration at 37 degrees C and up to a 40% at 45 degrees C were observed. Urine fortified with LSD and stored in amber glass or nontransparent polyethylene containers showed no change in concentration under any light conditions. Stability of LSD in transparent containers under light was dependent on the distance between the light source and the samples, the wavelength of light, exposure time, and the intensity of light. After prolonged exposure to heat in alkaline pH conditions, 10 to 15% of the parent LSD epimerized to iso-LSD. Under acidic conditions, less than 5% of the LSD was converted to iso-LSD. It was also demonstrated that trace amounts of metal ions in buffer or urine could catalyze the decomposition of LSD and that this process can be avoided by the addition of ]. | |||
| LSD shows significant ], with tolerance developing 24 hours after administration. The progression of tolerance at intervals shorter than 24 hours remains largely unknown.<ref>{{cite book |vauthors=Buchborn T, Grecksch G, Dieterich D, Hollt V |title=Neuropathology of Drug Addictions and Substance Misuse |doi=10.1016/B978-0-12-800212-4.00079-0 |chapter=Chapter 79 - Tolerance to Lysergic Acid Diethylamide: Overview, Correlates, and Clinical Implications |isbn=978-0-12-800212-4 |publisher=] |pages=848–849 |volume=2 |year=2016}}</ref> Tolerance typically resets to baseline after 3–4 days of abstinence.<ref name="pmid28701958">{{cite journal |title=A Single Dose of LSD Does Not Alter Gene Expression of the Serotonin 2A Receptor Gene (HTR2A) or Early Growth Response Genes (EGR1-3) in Healthy Subjects |vauthors=Dolder DS, Grünblatt E, Müller F, Borgwardt SJ, Liechti ME |date=28 June 2017 |doi=10.3389/fphar.2017.00423 |journal=Frontiers in Neuroscience |volume=8 |page=423 |pmid=28701958 |pmc=5487530 |doi-access=free}}</ref><ref>{{cite journal |journal=Pain Practice |page=455 |volume=23 |issue=4 |issn=1533-2500 |doi=10.1111/papr.13203 |doi-access=free |vauthors=Kooijman NI, Willegers T, Reuser A, Mulleners WM, Kramers C, ((Vissers KCP)), ((van der Wal SEI)) |date=4 January 2023 |pmid=36597700 |title=Are psychedelics the answer to chronic pain: A review of current literature| s2cid=255470638 |hdl=2066/291903 |hdl-access=free}}</ref> Significant cross-tolerance occurs between LSD, ] and ].<ref name="isbell_mescaline">{{cite journal |vauthors=Wolbach AB, Isbell H, Miner EJ |date=March 1962 |title=Cross tolerance between mescaline and LSD-25, with a comparison of the mescaline and LSD reactions |journal=Psychopharmacologia |volume=3 |pages=1–14 |pmid=14007904 |doi=10.1007/BF00413101 |s2cid=23803624 |url=http://www.erowid.org/references/refs_view.php?A=ShowDocPartFrame&C=ref&ID=2032&DocPartID=1893 |access-date=December 1, 2007 |archive-date=April 19, 2014 |archive-url=https://web.archive.org/web/20140419141528/http://www.erowid.org/references/refs_view.php?A=ShowDocPartFrame&C=ref&ID=2032&DocPartID=1893}}</ref><ref name="isbell_psilocybin">{{cite journal |vauthors=Isbell H, Wolbach AB, Wikler A, Miner EJ |title=Cross tolerance between LSD and psilocybin |journal=Psychopharmacologia |volume=2 |issue=3 |pages=147–159 |year=1961 |doi=10.1007/BF00407974 |url=http://www.erowid.org/references/refs_view.php?A=ShowDocPartFrame&C=ref&ID=1979&DocPartID=1843 |access-date=December 1, 2007 |pmid=13717955 |s2cid=7746880 |archive-date=March 15, 2016 |archive-url=https://web.archive.org/web/20160315102433/https://www.erowid.org/references/refs_view.php?a=showdocpartframe&c=ref&docpartid=1843&id=1979 |url-status=live }}</ref> A slight cross-tolerance to ] is observed in humans highly tolerant to LSD.<ref>{{cite journal |vauthors=Rosenberg D, Isbell H, Miner E, Logan C |doi=10.1007/BF00413244 |date=7 August 1963 |title=The effect of N,N-dimethyltryptamine in human subjects tolerant to lysergic acid diethylamide |journal=Psychopharmacologia |volume=5 |issue=3 |pages=223–224 |pmid=14138757 |s2cid=32950588}}</ref> Tolerance to LSD also builds up with consistent use,<ref name="springer">{{cite journal |vauthors=Jonas S, Downer JD |title=Gross behavioural changes in monkeys following administration of LSD-25, and development of tolerance to LSD-25 |journal=Psychopharmacologia |volume=6 |issue=4 |pages=303–386 |date=October 1964 |pmid=4953438 |doi=10.1007/BF00413161 |s2cid=11768927}}</ref> and is believed to result from serotonin 5-HT<sub>2A</sub> ].<ref name="pmid28701958" /> Researchers believe that tolerance returns to baseline after two weeks of not using psychedelics.<ref name=lsdtol>{{cite journal |vauthors=Schlemmer RF, Nawara C, Heinze WJ, Davis JM, Advokat C |title=Influence of environmental context on tolerance to LSD-induced behavior in primates |journal=Biological Psychiatry |volume=21 |issue=3 |pages=314–317 |date=March 1986 |pmid=3947713 |doi=10.1016/0006-3223(86)90053-3 |s2cid=35508993}}</ref> | |||
| ===Addiction and dependence liability=== | |||
| ==Production== | |||
| LSD is widely considered to be non-addictive, despite its potential for ].<ref name="NHM-MDMA">{{cite book |vauthors=Malenka RC, Nestler EJ, Hyman SE |veditors=Sydor A, Brown RY |title=Molecular Neuropharmacology: A Foundation for Clinical Neuroscience |year=2009 |publisher=McGraw-Hill Medical |location=New York |isbn=9780071481274 |pages=375 |edition=2nd |chapter=Chapter 15: Reinforcement and Addictive Disorders |quote=Several other classes of drugs are categorized as drugs of abuse but rarely produce compulsive use. These include psychedelic agents, such as lysergic acid diethylamide (LSD) |url=https://books.google.com/books?id=PjgfBQAAQBAJ |access-date=June 12, 2023 |archive-date=August 28, 2023 |archive-url=https://web.archive.org/web/20230828020503/https://books.google.com/books?id=PjgfBQAAQBAJ |url-status=live }}</ref><ref name="pmid14761703"/><ref name="Lus2006">{{cite journal |vauthors=Lüscher C, Ungless MA |title=The mechanistic classification of addictive drugs |journal=PLOS Medicine |volume=3 |issue=11 |pages=e437 |date=November 2006 |pmid=17105338 |pmc=1635740 |doi=10.1371/journal.pmed.0030437 |doi-access=free}}</ref><ref name="clinicalLSD"/> Attempts to train laboratory animals to ] LSD have been largely unsuccessful.<ref name="pmid14761703"/> Although tolerance to LSD builds up rapidly, a ] does not appear, suggesting that a potential syndrome does not necessarily relate to the possibility of acquiring rapid tolerance to a substance.<ref>{{cite journal |vauthors=Balestrieri A, Fontanari D |date=September 1959 |doi=10.1001/archpsyc.1959.03590030063008 |pages=279–282 |pmid=13796178 |title=Acquired and crossed tolerance to mescaline, LSD-25, and BOL-148|journal=Archives of General Psychiatry | volume=1 |issue=3}}</ref> A report examining ] for ] noted that almost no hallucinogens produced dependence, unlike psychoactive drugs of other classes such as ] and ]s.<ref name="pmid29366418">{{cite journal |journal=Current Neuropharmacology |volume=17 |issue=2 |pages=1–15 |title=Ayahuasca: Psychological and Physiologic Effects, Pharmacology and Potential Uses in Addiction and Mental Illness |doi=10.2174/1570159X16666180125095902 |doi-access=free |issn=1875-6190 |vauthors=Hamill J, Hallak J, Dursun SD, Baker G |year=2019 |pmid=29366418| pmc=6343205}}</ref><ref>{{cite journal |journal=Addiction |publisher=Society for the Study of Addiction |vauthors=Morgenstern J, Langenbucher J, Labouvie E |date=September 1994 |title=The generalizability of the dependence syndrome across substances: an examination of some properties of the proposed DSM-IV dependence criteria |volume=89 |issue=9 |pages=1105–1113 |doi=10.1111/j.1360-0443.1994.tb02787.x |pmid=7987187}}</ref> | |||
| ] | |||
| ===Cancer and pregnancy=== | |||
| Because an active dose of LSD is astonishingly minute, a large number of doses can be synthesized from a comparatively small amount of raw material. Beginning with ] ], for example, one can manufacture roughly one kilogram of pure, crystalline LSD from five kilograms of tartrate. Five kilograms of LSD — 25 kilograms of ergotamine tartrate — could provide 100 million "hits", sufficient for supplying the entire illicit demand of the United States. Since the masses involved are so small, concealing and transporting illicit LSD is much easier than smuggling other illegal drugs (say, ] or ]) in equal dosage quantities.<ref name="DEA-pub">"", DEA Publications.</ref> | |||
| The ] potential of LSD is unclear. Overall, the evidence seems to point to limited or no effect at commonly used doses.<ref>{{cite journal |vauthors=Li JH, Lin LF |title=Genetic toxicology of abused drugs: a brief review |journal=Mutagenesis |volume=13 |issue=6 |pages=557–65 |date=November 1998 |pmid=9862186 |doi=10.1093/mutage/13.6.557 |doi-access=free}}</ref> Studies showed no evidence of ] or mutagenic effects.<ref name=Pas2008/> | |||
| ==Overdose== | |||
| Manufacturing LSD requires laboratory equipment and experience in the field of ]. It takes two or three days to produce 30 to 100 grams of pure compound. It is believed that LSD usually is not produced in large quantities, but rather in a series of small batches. This technique minimizes the loss of precursor chemicals in case a synthesis step does not work as expected.<ref name="DEA-pub"/> | |||
| There have been no documented fatal human overdoses from LSD,<ref name="Pas2008"/><ref name="Lipow22">{{cite journal |journal=Transformative Medicine |title=NBOMe Toxicity and Fatalities: A Review of the Literature |volume=1 |issue=1 |date=March 2022 |vauthors=Lipow M, Kaleem SZ, Espiridion E |pages=12–18 |s2cid=247888583 |doi=10.54299/tmed/msot8578 |doi-access=free |issn=2831-8978}}</ref> although there has been no "comprehensive review since the 1950s" and "almost no legal clinical research since the 1970s".<ref name="Pas2008"/> Eight individuals who had accidentally consumed an exceedingly high amount of LSD, mistaking it for cocaine, and had gastric levels of 1000–7000 μg LSD tartrate per 100 mL and ] levels up to 26 μg/ml, had suffered from ], vomiting, respiratory problems, ], and light ]; however, all of them survived without residual effects upon hospital intervention.<ref name="Pas2008"/><ref>{{cite journal | vauthors = Klock JC, Boerner U, Becker CE | title = Coma, Hyperthermia and Bleeding Associated with Massive LSD Overdose: A Report of Eight Cases | journal = The Western Journal of Medicine | date = March 1974 | volume = 120 | issue = 3 | pages = 183–188 | pmid = 4816396 | pmc = 1129381}}</ref> | |||
| Individuals experiencing a bad trip after LSD intoxication may present with severe anxiety and tachycardia, often accompanied by phases of psychotic agitation and varying degrees of delusions.<ref name="pmid29408722" /> Cases of death on a bad trip have been reported due to ] (commonly known as a hogtie) and ] when the individuals were restrained by ].<ref name="pmid29408722" /> | |||
| ===Forms of LSD=== | |||
| ] | |||
| Massive doses are largely managed by ]s, and agitation can be addressed with ]s.<ref name="Medscape">{{EMedicine|article|1011615|LSD Toxicity Treatment & Management|treatment}}</ref><ref>{{cite journal |journal=Frontiers in Neuroscience |vauthors=Zawilska JB, Kacela M, Adamowicz P |doi=10.3389/fnins.2020.00078 |date=26 February 2020 |volume=14 |pmid=32174803 |title=NBOMes–Highly Potent and Toxic Alternatives of LSD |page=78 |pmc=7054380 |doi-access=free}}</ref> Reassurance in a ] is beneficial.<ref>{{Cite journal |vauthors=Hartogsohn I |date=2017 |title=Constructing drug effects: A history of set and setting |journal=Drug Science, Policy and Law |language=en |volume=3 |pages=205032451668332 |doi=10.1177/2050324516683325 |s2cid=53373205 |issn=2050-3245 |doi-access=free}}</ref> ] such as ] are not recommended as they may have adverse ].<ref name="Medscape" /> Gastrointestinal decontamination with ] is of little use due to the rapid absorption of LSD, unless done within 30–60 minutes of ingesting exceedingly huge amounts.<ref name="Medscape" /> Administration of ], ], and ] may be useful for treating ].<ref name="Medscape" /> | |||
| LSD is produced in crystalline form and then mixed with ]s or redissolved for production in ingestible forms. Liquid solution is either distributed as-is in small vials or, more commonly, sprayed onto or soaked into a distribution medium. Historically, LSD solutions were first sold on sugar cubes, but practical considerations forced a change to ] form. Early pills or tabs were flattened on both ends and identified by color: "grey flat", "blue flat", and so forth. Next came "domes", which were rounded on one end, then "double domes" rounded on both ends, and finally small tablets known as "microdots". Later still, LSD began to be distributed in thin squares of gelatin ("window panes", "gel tabs") and, most commonly, as blotter paper: sheets of paper impregnated with LSD and perforated into small squares of individual dosage units. The paper is then cut into small square pieces called "tabs" or "hits" for distribution. Individual producers often print designs onto the paper serving to identify different makers, batches or strengths, and such "blotter art" often emphasizes ] themes. | |||
| ===Designer drug overdose=== | |||
| LSD has been sold under a wide variety of street names including Acid, Trips, Alice Dee, 'Cid/Sid, Barrels, Blotter, Doses, Fry, "L", Liquid, Liquid A, Lucy, Lucy in the Sky with Diamonds, Microdots, Mind detergent, Orange cubes, Orange micro, ], Hits, Paper acid, Sacrament, ], Sugar, Sugar lumps, Sunshine, Tabs, Ticket, Twenty-five, Wedding bells, Windowpane, etc., as well as names that reflect the designs on the sheets of blotter paper.<ref name="erowid-faq">Honig, David. via ].</ref><ref>{{cite web | |||
| Many ] of ] (NBOMe) series, such as ] and ], are regularly sold as LSD in blotter papers.<ref name="pmid30261175">{{cite journal |journal=Biochemical Pharmacology |vauthors=Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Johnson RA, Janowsky A |title=Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors |doi=10.1016/j.bcp.2018.09.024 |pmid=30261175 |pmc=6298744 |volume=158 |pages=27–34 |date=December 2018}}</ref><ref>{{cite journal |journal=Journal of Analytical Toxicology |doi=10.1093/jat/bkv073 |pmc=4570937 |pmid=26378135 |title=Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and Other Dimethoxyphenyl-N-Ethanamine Derivatives on Blotter Paper |vauthors=Poklis JL, Raso SA, Alford KN, Poklis A, Peace MR |date=Oct 2015 |volume=39 |issue=8 |pages=617–623}}</ref> NBOMe compounds are often associated with life-threatening toxicity and death.<ref name="pmid30261175"/><ref name="pmid35343858">{{cite journal |journal=] |title=A cluster of 25B-NBOH poisonings following exposure to powder sold as lysergic acid diethylamide (LSD) |vauthors=Ivory ST, Rotella J, Schumann J, Greene SL |pages=966–969 |date=28 March 2022 |volume=60 |issue=8 |doi=10.1080/15563650.2022.2053150 |pmid=35343858 |s2cid=247764056}}</ref> Fatalities involved in NBOMe intoxication suggest that a significant number of individuals ingested the substance which they believed was LSD,<ref name="pmid31915427">{{cite journal |journal=Frontiers in Pharmacology |date=12 December 2019 |vauthors=Miliano C, Marti M, Pintori N, Castelli MP, Tirri M, Arfè R, De Luca MA |title=Neurochemical and Behavioral Profiling in Male and Female Rats of the Psychedelic Agent 25I-NBOMe |volume=10 |page=1406 |pmid=31915427 |pmc=6921684 |doi=10.3389/fphar.2019.01406 |doi-access=free}}</ref> and researchers report that "users familiar with LSD may have a false sense of security when ingesting NBOMe inadvertently".<ref name="Lipow22"/> Researchers state that the alleged physiological toxicity of LSD is likely due to psychoactive substances other than LSD.<ref name="pmid29408722"/> | |||
| | url = http://www.whitehousedrugpolicy.gov/streetterms/ByType.asp?intTypeID=6 | |||
| | title = Street Terms: Drugs and the Drug Trade | |||
| | publisher = ] | |||
| | date = ] | |||
| | accessdate = 2007-01-31}}</ref> On occasion, authorities have encountered the drug in other forms — including powder or crystal, and capsule. More than 200 types of LSD tablets have been encountered since 1969 and more than 350 paper designs have been observed since 1975. Designs range from simple five-point stars in black and white to exotic artwork in full four-color print. | |||
| NBOMe compounds are reported to have a bitter taste,<ref name="Lipow22"/> are not active orally,{{efn|The ] of ''N''-benzylphenethylamines via buccal, sublingual, or nasal absorption is 50–100 greater (by weight) than oral route compared to the parent ] compounds.<ref name="pmid24519542">{{cite journal |journal=Neurochemical Research |date=14 February 2014 |vauthors=Leth-Petersen S, Bundgaard C, Hansen M, Carnerup MA, Kehler J, Kristensen JL |title=Correlating the Metabolic Stability of Psychedelic 5-HT2A Agonists with Anecdotal Reports of Human Oral Bioavailability |volume=39 |issue=10 |pages=2018–2023 |doi=10.1007/s11064-014-1253-y |pmid=24519542| s2cid=254857910}}</ref> Researches hypothesize the low oral metabolic stability of ''N''-benzylphenethylamines is likely causing the low bioavailability on the oral route, although the metabolic profile of this compounds remains unpredictable; therefore researches state that the fatalities linked to these substances may partly be explained by differences in the metabolism between individuals.<ref name="pmid24519542"/>}} and are usually taken sublingually.<ref name="pmid28097528">{{cite book |title=Neuropharmacology of New Psychoactive Substances |vauthors=Halberstadt AL |doi=10.1007/7854_2016_64 |date=18 January 2017 |isbn=978-3-319-52444-3 |publisher=Springer |chapter=Pharmacology and Toxicology of N-Benzylphenethylamine ("NBOMe") Hallucinogens |series=Current Topics in Behavioral Neurosciences |volume=32 |pages=283–311 |pmid=28097528}}</ref> When NBOMes are administered sublingually, ] of the tongue and mouth followed by a metallic chemical taste was observed, and researchers describe this physical side effect as one of the main discriminants between NBOMe compounds and LSD.<ref>{{cite journal |vauthors=Duffau B, Camargo C, Kogan M, Fuentes E, Kennedy Cassels B |journal=Journal of Chromatographic Science |volume=54 |issue=7 |date=August 2016 |pages=1153–1158 |title=Analysis of 25 C NBOMe in Seized Blotters by HPTLC and GC–MS |pmc=4941995 |pmid=27406128 |doi=10.1093/chromsci/bmw095 |doi-access=free}}</ref><ref>{{cite journal |pmid=25105138 |pmc=4106087 |doi=10.1155/2014/734749 |title=25C-NBOMe: preliminary data on pharmacology, psychoactive effects, and toxicity of a new potent and dangerous hallucinogenic drug |journal=BioMed Research International |date=3 July 2014 |vauthors=Francesco SB, Ornella C, Gabriella A, Giuseppe V, Rita S, Flaminia BP, Eduardo C, Pierluigi S, Giovanni M, Guiseppe B, Fabrizio S |volume=2014 |page=734749 |doi-access=free}}</ref><ref>{{cite book |title=Novel Psychoactive Substances: Classification, Pharmacology and Toxicology |chapter=Pharmacology and toxicology of N-Benzyl-phenylethylamines (25X-NBOMe) hallucinogens |vauthors=Potts AJ, ((Thomas SHL)), Hill SL |veditors=Dargan P, Wood D |doi=10.1016/B978-0-12-818788-3.00008-5 |isbn=978-0-12-818788-3 |pages=279–300 |edition=2nd |publisher=Academic Press |date=September 2021 |s2cid=240583877}}</ref> Despite its high potency, recreational doses of LSD have only produced low incidents of acute toxicity, but NBOMe compounds have extremely different safety profiles.<ref name="Lipow22"/><ref name="pmid35343858"/> Testing with ] gives a positive result for LSD and a negative result for NBOMe compounds.<ref>{{Cite journal | vauthors = Díaz Moreno M, Alarcón Ayala N, Estrada Y, Morris V, Quintero J |date=November 2022 |title=Échele Cabeza as a harm reduction project and activist movement in Colombia |url=https://www.emerald.com/insight/content/doi/10.1108/DHS-07-2022-0026/full/html |journal=] |language=en |volume=23 |issue=3 |pages=263–276 |doi=10.1108/DHS-07-2022-0026 |issn=2752-6739}}</ref><ref>{{cite journal | vauthors = Clancy L, Philp M, Shimmon R, Fu S | title = Development and validation of a color spot test method for the presumptive detection of 25-NBOMe compounds | journal = Drug Testing and Analysis | volume = 13 | issue = 5 | pages = 929–943 | date = May 2021 | pmid = 32744773 | doi = 10.1002/dta.2905 }}</ref> | |||
| == Legal status == | |||
| The ] ] (adopted in 1971) requires its parties to prohibit LSD. Hence, it is illegal in all parties to the convention, which includes the ], ], and most of ]. However, enforcement of extant laws varies from country to country. | |||
| ==Pharmacology== | |||
| LSD is easy to conceal and smuggle. A tiny vial can contain thousands of doses. Not much money is made from retail-level sales of LSD, so the drug is typically not associated with the violent ] organizations involved in ] and ] smuggling. | |||
| === |
===Pharmacodynamics=== | ||
| ] (K<sub>i</sub>) of LSD for various ]s. The lower the value, the more strongly LSD binds to that receptor (i.e., with higher affinity). The horizontal line represents an approximate value for human plasma concentrations of LSD, and hence, affinities that are above the line are unlikely to be involved in LSD's effects. Values are averages the ].<ref name="PDSPKiDatabase" />]] | |||
| In Canada, LSD is a controlled substance under Schedule III of the ] (CDSA). Every person who seeks to obtain the substance without disclosing authorization to obtain such substances 30 days prior to obtaining another prescription from a practitioner is guilty of an indictable offence and liable to imprisonment for a term not exceeding 3 years. Possession for purpose of trafficking is guilty of an indictable offence and liable to imprisonment for 10 years. | |||
| {| class="wikitable floatright" style="font-size:small;" | |||
| ===Hong Kong=== | |||
| |+ {{Nowrap|Activities of LSD}} | |||
| In ], Lysergide and derivatives are regulated under Schedule 1 of ] Chapter 134 ''Dangerous Drugs Ordinance'', and can only be used legally by health professionals and for university research purposes. The substance can be given by pharmacists under a prescription. Anyone who supplies the substance without presciption can be fined $10000(HKD). The penalty for trafficking or illegally manufacturing the substance is a $5,000,000 (]) fine and life imprisonment. Possession of the substance for consumption without license from the Department of Health is illegal with a $1,000,000 (HKD) fine and/or 7 years' imprisonment. | |||
| |- | |||
| ! ] !! ] (K<sub>i</sub>, nM) | |||
| |- | |||
| | ] || 0.64–7.3 | |||
| |- | |||
| | ] || 3.9 | |||
| |- | |||
| | ] || 3.9–14 | |||
| |- | |||
| | ] || 93 | |||
| |- | |||
| | ] || {{Abbr|ND|No data}} | |||
| |- | |||
| | ] || 0.48–21 (K<sub>i</sub>)<br />0.24–225 ({{Abbrlink|EC<sub>50</sub>|half-maximal effective concentration}})<br />23–84% ({{Abbrlink|E<sub>max</sub>|maximal efficacy}}) | |||
| |- | |||
| | ] || 0.98–30 (K<sub>i</sub>)<br />8.9–12,000 ({{Abbr|EC<sub>50</sub>|half-maximal effective concentration}})<br />13–71% ({{Abbr|E<sub>max</sub>|maximal efficacy}}) | |||
| |- | |||
| | ] || 1.1–48 (K<sub>i</sub>)<br />0.85 ({{Abbr|EC<sub>50</sub>|half-maximal effective concentration}})<br />26–79% ({{Abbr|E<sub>max</sub>|maximal efficacy}}) | |||
| |- | |||
| | ] || >10,000 | |||
| |- | |||
| | ] || 1,000 (rat) | |||
| |- | |||
| | ] || 9.0 | |||
| |- | |||
| | ] || 3.2 (rat) | |||
| |- | |||
| | ] || 2.3–6.9 | |||
| |- | |||
| | ] || 6.3–6.6 | |||
| |- | |||
| | ] || 670–1,128 | |||
| |- | |||
| | ] || 8,677 | |||
| |- | |||
| | ] || {{Abbr|ND|No data}} | |||
| |- | |||
| | ] || 12–46 | |||
| |- | |||
| | ] || {{Abbr|ND|No data}} | |||
| |- | |||
| | ] || {{Abbr|ND|No data}} | |||
| |- | |||
| | ] || 140–1,601 | |||
| |- | |||
| | ] || 740–3,461 | |||
| |- | |||
| | ] || {{Abbr|ND|No data}} | |||
| |- | |||
| | ] || 177–340 | |||
| |- | |||
| | ] || 110–126 | |||
| |- | |||
| | ] || 27 | |||
| |- | |||
| | ] || 56–158 | |||
| |- | |||
| | ] || 344 | |||
| |- | |||
| | ] || 1,100–1,540 | |||
| |- | |||
| | ]–] || {{Abbr|ND|No data}} | |||
| |- | |||
| | ]–] || {{Abbr|ND|No data}} | |||
| |- | |||
| | ] || {{Abbr|ND|No data}} | |||
| |- | |||
| | ] || {{Abbr|ND|No data}} | |||
| |- | |||
| | ] || {{Abbr|ND|No data}} | |||
| |- | |||
| | ] || 450 (K<sub>i</sub>) (rat)<br />10,000 (K<sub>i</sub>) (mouse)<br />1,400 ({{Abbr|EC<sub>50</sub>|half-maximal effective concentration}}) (rat)<br />9,700 ({{Abbr|EC<sub>50</sub>|half-maximal effective concentration}}) (mouse)<br />>20,000 ({{Abbr|EC<sub>50</sub>|half-maximal effective concentration}}) (human) | |||
| |- | |||
| | {{Abbrlink|SERT|Serotonin transporter}} || >30,000 (K<sub>i</sub>)<br />>100,000 ({{Abbrlink|IC<sub>50</sub>|half-maximal inhibitory concentration}}) | |||
| |- | |||
| | {{Abbrlink|NET|Norepinephrine transporter}} || 5,600–>30,000 (K<sub>i</sub>)<br />>100,000 ({{Abbr|IC<sub>50</sub>|half-maximal inhibitory concentration}}) | |||
| |- | |||
| | {{Abbrlink|DAT|Dopamine transporter}} || >30,000 (K<sub>i</sub>)<br />>100,000 ({{Abbr|IC<sub>50</sub>|half-maximal inhibitory concentration}}) | |||
| |- class="sortbottom" | |||
| | colspan="2" style="width: 1px; background-color:#eaecf0; text-align: center;" | '''Notes:''' The smaller the value, the more avidly the drug binds to the site. All proteins are human unless otherwise noted. '''Refs:''' <ref name="PDSPKiDatabase">{{cite web | title=PDSP Database | website=UNC | url=https://pdsp.unc.edu/databases/pdsp.php?testFreeRadio=testFreeRadio&testLigand=lsd&kiAllRadio=all&doQuery=Submit+Query | language=zu | access-date=11 December 2024}}</ref><ref name="BindingDB">{{cite web | vauthors = Liu T | title=BindingDB BDBM21342 (4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclohexadeca-1(16),2,9,12,14-pentaene-4-carboxamide::CHEMBL263881::LSD::LSD 25::LSD,(+)::LSD,l-::Lysergic Acid Diethylamide::Lysergic Acid Diethylamide Tartrate::US20240166618, Compound LSD::-LSD::d-Isolysergic acid amide | website=BindingDB | url=https://www.bindingdb.org/rwd/bind/chemsearch/marvin/MolStructure.jsp?monomerid=21342 | access-date=11 December 2024}}</ref><ref name="HolzeSinghLiechti2024">{{cite journal | vauthors = Holze F, Singh N, Liechti ME, D'Souza DC | title = Serotonergic Psychedelics: A Comparative Review of Efficacy, Safety, Pharmacokinetics, and Binding Profile | journal = Biol Psychiatry Cogn Neurosci Neuroimaging | volume = 9 | issue = 5 | pages = 472–489 | date = May 2024 | pmid = 38301886 | doi = 10.1016/j.bpsc.2024.01.007 | url = | doi-access = free }}</ref><ref name="Ray2010">{{cite journal | vauthors = Ray TS | title = Psychedelics and the human receptorome | journal = PLOS ONE | volume = 5 | issue = 2 | pages = e9019 | date = February 2010 | pmid = 20126400 | pmc = 2814854 | doi = 10.1371/journal.pone.0009019 | doi-access = free | bibcode = 2010PLoSO...5.9019R | url = }}</ref><ref name="RickliLuethiReinisch2015">{{cite journal | vauthors = Rickli A, Luethi D, Reinisch J, Buchy D, Hoener MC, Liechti ME | title = Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs) | journal = Neuropharmacology | volume = 99 | issue = | pages = 546–553 | date = December 2015 | pmid = 26318099 | doi = 10.1016/j.neuropharm.2015.08.034 | url = http://edoc.unibas.ch/56163/1/20170921163006_59c3cceeb8e5d.pdf}}</ref><ref name="RickliMoningHoener2016">{{cite journal | vauthors = Rickli A, Moning OD, Hoener MC, Liechti ME | title = Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens | journal = Eur Neuropsychopharmacol | volume = 26 | issue = 8 | pages = 1327–1337 | date = August 2016 | pmid = 27216487 | doi = 10.1016/j.euroneuro.2016.05.001 | url = http://edoc.unibas.ch/53326/1/20170117174852_587e4af45b658.pdf}}</ref><br /><ref name="LuethiTrachselHoener2018">{{cite journal | vauthors = Luethi D, Trachsel D, Hoener MC, Liechti ME | title = Monoamine receptor interaction profiles of 4-thio-substituted phenethylamines (2C-T drugs) | journal = Neuropharmacology | volume = 134 | issue = Pt A | pages = 141–148 | date = May 2018 | pmid = 28720478 | doi = 10.1016/j.neuropharm.2017.07.012 | url = https://edoc.unibas.ch/57358/1/20170920150712_59c2680084ec5.pdf}}</ref><ref name="JanowskyEshlemanJohnson2014">{{cite journal | vauthors = Janowsky A, Eshleman AJ, Johnson RA, Wolfrum KM, Hinrichs DJ, Yang J, Zabriskie TM, Smilkstein MJ, Riscoe MK | title = Mefloquine and psychotomimetics share neurotransmitter receptor and transporter interactions in vitro | journal = Psychopharmacology (Berl) | volume = 231 | issue = 14 | pages = 2771–2783 | date = July 2014 | pmid = 24488404 | pmc = 4097020 | doi = 10.1007/s00213-014-3446-0 | url = }}</ref><ref name="Wsół2023">{{cite journal | vauthors = Wsół A | title = Cardiovascular safety of psychedelic medicine: current status and future directions | journal = Pharmacol Rep | volume = 75 | issue = 6 | pages = 1362–1380 | date = December 2023 | pmid = 37874530 | pmc = 10661823 | doi = 10.1007/s43440-023-00539-4 | url = }}</ref><ref name="EganGrindeDupre2000">{{cite journal | vauthors = Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, Herrick-Davis K | title = Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT(2A) and 5-HT(2C) receptors | journal = Synapse | volume = 35 | issue = 2 | pages = 144–150 | date = February 2000 | pmid = 10611640 | doi = 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K | url = }}</ref><ref name="PorterBenwellLamb1999">{{cite journal | vauthors = Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ | title = Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells | journal = Br J Pharmacol | volume = 128 | issue = 1 | pages = 13–20 | date = September 1999 | pmid = 10498829 | pmc = 1571597 | doi = 10.1038/sj.bjp.0702751 | url = }}</ref><ref name="GainetdinovHoenerBerry2018">{{cite journal | vauthors = Gainetdinov RR, Hoener MC, Berry MD | title = Trace Amines and Their Receptors | journal = Pharmacol Rev | volume = 70 | issue = 3 | pages = 549–620 | date = July 2018 | pmid = 29941461 | doi = 10.1124/pr.117.015305 | url = }}</ref><ref name="SimmlerBuchyChaboz2016">{{cite journal | vauthors = Simmler LD, Buchy D, Chaboz S, Hoener MC, Liechti ME | title = In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1 | journal = J Pharmacol Exp Ther | volume = 357 | issue = 1 | pages = 134–144 | date = April 2016 | pmid = 26791601 | doi = 10.1124/jpet.115.229765 | url = }}</ref> | |||
| |} | |||
| Most ]s are not significantly ], and LSD is therefore atypical in this regard. The agonism of the ] by LSD may contribute to its psychoactive effects in humans.<ref name="nichols_closes_shop">{{cite journal | vauthors = Marona-Lewicka D, Thisted RA, Nichols DE | title = Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis | journal = Psychopharmacology | volume = 180 | issue = 3 | pages = 427–35 | date = July 2005 | pmid = 15723230 | doi = 10.1007/s00213-005-2183-9 | s2cid = 23565306 }}</ref> | |||
| ===United States: Prior to 1967=== | |||
| {{Further|]}} | |||
| Beginning in the 1950's the ] began a research program code named ]. Experiments included administering LSD to CIA employees, military personnel, doctors, other government agents, prostitutes, mentally ill patients, and members of the general public in order to study their reactions, usually without the subject's knowledge. The project was revealed in the US congressional ]. | |||
| LSD binds to most serotonin receptor subtypes except for the ] and ]s. However, most of these receptors are affected at too low affinity to be sufficiently activated by the brain concentration of approximately 10–20 ].<ref name="pmid14761703"/> In humans, recreational doses of LSD can affect ] (K<sub>i</sub> = 1.1 nM), ] (K<sub>i</sub> = 2.9 nM), ] (K<sub>i</sub> = 4.9 nM), ] (K<sub>i</sub> = 23 nM), ] (K<sub>i</sub> = 9 nM ), and ] (K<sub>i</sub> = 2.3 nM).<ref name="Aghajanian" /> Although not present in humans, ] found in rodents also have a high affinity for LSD.<ref>{{cite journal |vauthors=Nelson DL |title=5-HT5 receptors |journal=Current Drug Targets. CNS and Neurological Disorders |volume=3 |issue=1 |pages=53–58 |date=February 2004 |pmid=14965244 |doi=10.2174/1568007043482606}}</ref> The psychedelic effects of LSD are attributed to ] of 5-HT<sub>2A</sub> ].<ref>{{cite journal |vauthors=Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J |title=Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists |journal=Neuroscience Letters |volume=493 |issue=3 |pages=76–79 |date=April 2011 |pmid=21276828 |pmc=3064746 |doi=10.1016/j.neulet.2011.01.046}}</ref> Many but not all 5-HT<sub>2A</sub> ]s are ] and 5-HT<sub>2A</sub> ] block the psychedelic activity of LSD. LSD exhibits ] at the 5-HT<sub>2A</sub> and 5-HT<sub>2C</sub> receptors in that it activates the ] enzyme ] instead of activating the enzyme ] as the endogenous ligand serotonin does.<ref>{{cite journal |vauthors=Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB |title=Functional selectivity and classical concepts of quantitative pharmacology |journal=The Journal of Pharmacology and Experimental Therapeutics |volume=320 |issue=1 |pages=1–13 |date=January 2007 |pmid=16803859 |doi=10.1124/jpet.106.104463 |url=https://jpet.aspetjournals.org/content/320/1/1 |s2cid=447937 |access-date=June 11, 2023 |archive-date=June 11, 2023 |archive-url=https://web.archive.org/web/20230611010342/https://jpet.aspetjournals.org/content/320/1/1 |url-status=live }}</ref> | |||
| Prior to October 6th, 1966, LSD was available legally in the United States as an experimental ] drug. (LSD "apostle" ] actively promoted the drug between the 1950s and the 1970s and introduced thousands of people to it.) The ] classified it as a ] drug according to the ] of 1970. As such, the ] holds that LSD meets the following three criteria: it is deemed to have a high potential for abuse; it has no legitimate medical use in treatment; and there is a lack of accepted safety for its use under medical supervision. (LSD prohibition does not make an exception for religious use.) Lysergic acid and lysergic acid amide, LSD precursors, are both classified in ] of the Controlled Substances Act. Ergotamine tartrate, a precursor to lysergic acid, is regulated under the ]. | |||
| Exactly how LSD produces its effects is unknown, but it is thought that it works by increasing ] release in the ]<ref name="pmid14761703"/> and therefore ] in this area, specifically in ].<ref>{{cite journal | vauthors = Aghajanian GK, Marek GJ | title = Serotonin and hallucinogens | journal = Neuropsychopharmacology | volume = 21 | issue = 2 Suppl | pages = 16S–23S | date = August 1999 | pmid = 10432484 | doi = 10.1016/S0893-133X(98)00135-3 | doi-access = free }}</ref> LSD, like many other drugs of recreational use, has been shown to activate ]-related pathways.<ref>{{cite journal | vauthors = Svenningsson P, Nairn AC, Greengard P | title = DARPP-32 mediates the actions of multiple drugs of abuse | journal = The AAPS Journal | volume = 7 | issue = 2 | pages = E353-60 | date = October 2005 | pmid = 16353915 | pmc = 2750972 | doi = 10.1208/aapsj070235 }}</ref> The drug enhances dopamine D<sub>2</sub> receptor ] recognition and ] of D<sub>2</sub>–5-HT<sub>2A</sub> receptor complexes,<ref name="pmid24309097">{{cite journal | vauthors = Borroto-Escuela DO, Romero-Fernandez W, Narvaez M, Oflijan J, Agnati LF, Fuxe K | title = Hallucinogenic 5-HT2AR agonists LSD and DOI enhance dopamine D2R protomer recognition and signaling of D2-5-HT2A heteroreceptor complexes | journal = Biochemical and Biophysical Research Communications | volume = 443 | issue = 1 | pages = 278–84 | date = January 2014 | pmid = 24309097 | doi = 10.1016/j.bbrc.2013.11.104 }}</ref> which may contribute to its psychotropic effects.<ref name="pmid24309097" /> LSD has been shown to have low affinity for ], displaying antihistamine effects.<ref>{{cite journal | vauthors = Green JP, Johnson CL, Weinstein H, Maayani S | title = Antagonism of histamine-activated adenylate cyclase in brain by D-lysergic acid diethylamide | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 74 | issue = 12 | pages = 5697–701 | date = December 1977 | pmid = 23536 | pmc = 431860 | doi = 10.1073/pnas.74.12.5697 | bibcode = 1977PNAS...74.5697G | doi-access = free }}</ref><ref name="synth2"/> | |||
| LSD has been manufactured illegally since the 1960s. Historically, LSD was distributed not for profit, but because those who made and distributed it truly believed that the psychedelic experience could do good for humanity, that it expanded the mind and could bring understanding and love. A limited number of chemists, probably fewer than a dozen, are believed to have manufactured nearly all of the illicit LSD available in the United States. The best known of these is undoubtedly ], usually known simply as Owsley. The former chemistry student set up a private LSD lab in the mid-Sixties in ] and supplied the LSD consumed at the famous ] parties held by ] and his ], and other major events such as the ] in San Francisco in January 1967. He also had close social connections to leading San Francisco bands the ], ] and ], regularly supplied them with his LSD and also worked as their live sound engineer and made many tapes of these groups in concert. Owsley's LSD activities — immortalized by ] in their song "]" — ended with his arrest at the end of 1967, but some other manufacturers probably operated continuously for 30 years or more. Announcing Owsley's first bust in 1966, The ]'s headline "LSD Millionaire Arrested" inspired the rare Grateful Dead song "Alice D. Millionaire." | |||
| LSD is a ] that induces a conformation in serotonin receptors that preferentially recruits ] over ]s.<ref name="Chen_2017">{{cite journal |vauthors=Chen Q, Tesmer JJ |title=A Receptor on Acid |journal=Cell |volume=168 |issue=3 |pages=339–341 |date=January 2017 |doi=10.1016/j.cell.2017.01.012 |pmid=28129534 |pmc=5520807}}</ref> LSD also has an exceptionally long ] when bound to serotonin receptors lasting hours, consistent with the long-lasting effects of LSD despite its relatively rapid ].<ref name="Chen_2017" /> A crystal structure of 5-HT<sub>2B</sub> bound to LSD reveals an extracellular loop that forms a lid over the diethylamide end of the binding cavity which explains the slow rate of LSD unbinding from serotonin receptors.<ref name="WackerWang2017">{{cite journal | vauthors = Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, Lansu K, Schools ZL, Che T, Nichols DE, Shoichet BK, Dror RO, Roth BL | title = Crystal Structure of an LSD-Bound Human Serotonin Receptor | journal = Cell | volume = 168 | issue = 3 | pages = 377–389.e12 | date = January 2017 | pmid = 28129538 | pmc = 5289311 | doi = 10.1016/j.cell.2016.12.033 }}</ref> The related ] ] (LSA) that lacks the diethylamide ] is far less hallucinogenic in comparison.<ref name="WackerWang2017" /> | |||
| ===United States: 1970 to the present=== | |||
| ] | |||
| LSD, like other psychedelics, has been found to increase the expression of genes related to synaptic plasticity.<ref>{{cite journal | vauthors = Calder AE, Hasler G | title = Towards an understanding of psychedelic-induced neuroplasticity | journal = Neuropsychopharmacology | volume = 48 | issue = 1 | pages = 104–112 | date = January 2023 | pmid = 36123427 | pmc = 9700802 | doi = 10.1038/s41386-022-01389-z }}</ref> This is in part due to binding to brain-derived neurotrophic factor (BDNF) receptor TrkB.<ref>{{cite journal | vauthors = Moliner R, Girych M, Brunello CA, Kovaleva V, Biojone C, Enkavi G, Antenucci L, Kot EF, Goncharuk SA, Kaurinkoski K, Kuutti M, Fred SM, Elsilä LV, Sakson S, Cannarozzo C, Diniz CR, Seiffert N, Rubiolo A, Haapaniemi H, Meshi E, Nagaeva E, Öhman T, Róg T, Kankuri E, Vilar M, Varjosalo M, Korpi ER, Permi P, Mineev KS, Saarma M, Vattulainen I, Casarotto PC, Castrén E | title = Psychedelics promote plasticity by directly binding to BDNF receptor TrkB | journal = Nature Neuroscience | volume = 26 | issue = 6 | pages = 1032–1041 | date = June 2023 | pmid = 37280397 | doi = 10.1038/s41593-023-01316-5 | pmc = 10244169 }}</ref> | |||
| American LSD usage declined in the 1970s and 1980s, then experienced a mild resurgence in popularity in the 1990s. Although there were many distribution channels during this decade, the U.S. ] identified continued tours by the ] band ] and the then-burgeoning ] scene as primary venues for LSD trafficking and consumption. American LSD usage fell sharply circa 2000. The decline is attributed to the arrest of two chemists, ], a Harvard-educated organic chemist, and ]. According to DEA reports, black market LSD availability dropped by 95% after the two were arrested in 2000. These arrests were a result of the largest LSD manufacturing raid in DEA history.<ref>Seper, Jerry. "". ''The Washington Times'' 27 November 2003.</ref> | |||
| ====Mechanisms of action==== | |||
| Pickard was an alleged member of the ] group that produced and sold LSD in California during the late 1960s and early 1970s. It is believed he had links to other "cooks" associated with this group — an original source of the drug back in the 1960s — and his arrest may have forced other operations to cease production, leading to the large decline in street availability. | |||
| {{Multiple image | |||
| | align = left | |||
| | total_width = 500 | |||
| | image1 = FMRI V1 RSFC LSD.png | |||
| | caption1 = ] ] shows increased ] (V1) ] (CBF) and increased V1 ] (RSFC), which correlated more strongly with the visual hallucinatory aspect of the LSD experience. Increased V1 RSFC also correlated with ] (VAS) ratings of simple hallucinations and the magnitude of CBF observed in the visual cortex correlated positively with ratings of complex imagery on the LSD-induced ] (ASC).<ref name="pmid27071089">{{cite journal |vauthors=Carhart-Harris RL, Muthukumaraswamy S, Roseman L, Kaelen M, Droog W, Murphy K, Tagliazucchi E, Schenberg EE, Nest T, Orban C, Leech R, Williams LT, Williams TM, Bolstridge M, Sessa B, McGonigle J, Sereno MI, Nichols D, Hellyer PJ, Hobden P, Evans J, Singh KD, Wise RG, Curran HV, Feilding A, Nutt DJ |title=Neural correlates of the LSD experience revealed by multimodal neuroimaging |journal=] |volume=113 |issue=17 |pages=4853–4858 |date=11 April 2016 |pmid=27071089 |pmc=4855588 |doi=10.1073/pnas.1518377113 |bibcode=2016PNAS..113.4853C |doi-access=free}}</ref> | |||
| | width1 = | |||
| | height1 = | |||
| | image2 = FMRI PH RSFC LSD.png | |||
| | caption2 = ] ] shows decreased ] (PH) ] (RSFC), which correlated with the ] aspect of the LSD experience. A significant relationship was also found between decreased ] (PCC) ] and ] (DMN) disintegration with ego-dissolution.<ref name="pmid27071089" /> | |||
| | width2 = | |||
| | height2 = | |||
| | footer = | |||
| }} | |||
| Neuroimaging studies using ] ] recently suggested that LSD changes the cortical functional architecture.<ref name="Singleton SP">{{cite journal | vauthors = Singleton SP, Luppi AI, Carhart-Harris RL, Cruzat J, Roseman L, Nutt DJ, Deco G, Kringelbach ML, Stamatakis EA, Kuceyeski A |title = Receptor-informed network control theory links LSD and psilocybin to a flattening of the brain's control energy landscape|journal = Nat Commun |volume=13 | issue=1 | page=5812 | date=Oct 2022 | pmid=36192411 | doi = 10.1038/s41467-022-33578-1 | pmc=9530221 | bibcode=2022NatCo..13.5812S | doi-access=free }}</ref> These modifications spatially overlap with the distribution of serotoninergic receptors. In particular, increased connectivity and activity were observed in regions with high expression of ] receptor, while a decrease in activity and connectivity was observed in cortical areas that are dense with ] receptor.<ref name="Delli Pizzi S BP:CNNI">{{cite journal |vauthors=Delli Pizzi S, Chiacchiaretta P, Sestieri C, Ferretti A, Onofrj M, Della Penna S, Roseman L, Timmermann C, Nutt DJ, Carhart-Harris RL, Sensi SL |title=Spatial Correspondence of LSD-Induced Variations on Brain Functioning at Rest With Serotonin Receptor Expression |journal=Biol Psychiatry Cogn Neurosci Neuroimaging |volume=8 |issue=7 |pages=768–776 |date=July 2023 |pmid=37003409 |doi=10.1016/j.bpsc.2023.03.009 |s2cid=257862535}}</ref> Experimental data suggest that subcortical structures, particularly the thalamus, play a synergistic role with the cerebral cortex in mediating the psychedelic experience. LSD, through its binding to cortical ] receptor, may enhance excitatory neurotransmission along frontostriatal projections and, consequently, reduce thalamic filtering of sensory stimuli towards the cortex.<ref name="Delli Pizzi S NeuroImage">{{cite journal |vauthors=Delli Pizzi S, Chiacchiaretta P, Sestieri C, Ferretti A, Tullo MG, Della Penna S, Martinotti G, Onofrj M, Roseman L, Timmermann C, Nutt DJ, Carhart-Harris RL, Sensi SL |title=LSD-induced changes in the functional connectivity of distinct thalamic nuclei |journal=NeuroImage |volume=283 |page=120414 |date=Dec 2023 |pmid=37858906 |doi=10.1016/j.neuroimage.2023.120414 |doi-access=free}}</ref> This phenomenon appears to selectively involve ventral, intralaminar, and pulvinar nuclei.<ref name="Delli Pizzi S NeuroImage"/> | |||
| The DEA claims these two individuals were responsible for the vast majority of LSD sold illegally in the United States and a significant amount of the LSD sold in ], and that they worked closely with organized traffickers. While this claim may have some bearing, the extent of Pickard's direct influence on the overall availability in the United States is not fully known. Some attest that "Pickard's Acid" was sold exclusively in Europe, and was not distributed through American music venues. | |||
| ===Pharmacokinetics=== | |||
| In November of 2003, Pickard was sentenced to life imprisonment without parole, and Apperson was sentenced to 30 years imprisonment without parole, after being convicted in ] of running a large scale LSD manufacturing operation out of several clandestine laboratories, including a former ] near ]. | |||
| The acute effects of LSD normally last between 6 and 10 hours depending on dosage, tolerance, and age.<ref name="tihkal">{{cite book |vauthors=Shulgin A, Shulgin A |author-link1=Alexander Shulgin |author-link2=Ann Shulgin |chapter-url=http://www.erowid.org/library/books_online/tihkal/tihkal26.shtml |archive-date=15 October 2008 |archive-url=http://archive.wikiwix.com/cache/20081015082653/http://www.erowid.org/library/books_online/tihkal/tihkal26.shtml |chapter=LSD |title=] |location=Berkeley, CA |publisher=Transform Press |date=1997 |isbn=0-9630096-9-9}}</ref><ref>{{cite journal | vauthors = Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A | title = The pharmacology of lysergic acid diethylamide: a review | journal = CNS Neuroscience & Therapeutics | volume = 14 | issue = 4 | pages = 295–314 | date = 2008-11-11 | pmid = 19040555 | pmc = 6494066 | doi = 10.1111/j.1755-5949.2008.00059.x }}</ref> Aghajanian and Bing (1964) found LSD had an elimination half-life of only 175 minutes (about 3 hours).<ref name="Aghajanian">{{cite journal |vauthors=Aghajanian GK, Bing OH |title=Persistence of lysergic acid diethylamide in the plasma of human subjects |journal=Clinical Pharmacology and Therapeutics |volume=5 |issue=5 |pages=611–614 |year=1964 |pmid=14209776 |doi=10.1002/cpt196455611| url=http://www.maps.org/w3pb/new/1964/1964_aghajanian_2224_1.pdf |s2cid=29438767 |archive-url= https://web.archive.org/web/20090327144227/http://www.maps.org/w3pb/new/1964/1964_aghajanian_2224_1.pdf |archive-date=March 27, 2009}}</ref> However, using more accurate techniques, Papac and Foltz (1990) reported that 1 μg/kg oral LSD given to a single male volunteer had an apparent plasma half-life of 5.1 hours, with a peak plasma concentration of 5 ng/mL at 3 hours post-dose.<ref name="Papac">{{cite journal |vauthors=Papac DI, Foltz RL |title=Measurement of lysergic acid diethylamide (LSD) in human plasma by gas chromatography/negative ion chemical ionization mass spectrometry |journal=Journal of Analytical Toxicology |volume=14 |issue=3 |pages=189–190 |date=May–June 1990 |pmid=2374410 |doi=10.1093/jat/14.3.189 |url=http://www.erowid.org/references/refs_view.php?A=ShowDocPartFrame&C=ref&ID=6265&DocPartID=6624 |url-status=live|format=PDF |archive-date=April 29, 2011|archive-url=https://web.archive.org/web/20110429060433/http://www.erowid.org/references/refs_view.php?A=ShowDocPartFrame&C=ref&ID=6265&DocPartID=6624}}</ref> | |||
| The ] of LSD were not properly determined until 2015, which is not surprising for a drug with the kind of low-μg potency that LSD possesses.<ref name=Dol2015 /><ref name=Muc2016 /> In a sample of 16 healthy subjects, a single mid-range 200 μg oral dose of LSD was found to produce mean ]s of 4.5 ng/mL at a median of 1.5 hours (range 0.5–4 hours) post-administration.<ref name=Dol2015 /><ref name=Muc2016 /> Concentrations of LSD decreased following ] with a ] of 3.6±0.9 hours and a ] of 8.9±5.9 hours.<ref name=Dol2015 /><ref name=Muc2016 /> | |||
| LSD manufacturers and traffickers can be categorized into two groups: A few large scale producers, such as the aforementioned Pickard and Apperson, and an equally limited number of small, clandestine chemists, consisting of independent producers who, operating on a comparatively limited scale, can be found throughout the country. As a group, independent producers are of less concern to the ] than the larger groups, as their product reaches only local markets. | |||
| The effects of the dose of LSD given lasted for up to 12 hours and were closely correlated with the concentrations of LSD present in circulation over time, with no acute ] observed.<ref name=Dol2015 /><ref name=Muc2016 /> Only 1% of the drug was eliminated in ] unchanged, whereas 13% was eliminated as the major ] 2-oxo-3-hydroxy-LSD (O-H-LSD) within 24 hours.<ref name=Dol2015 /><ref name=Muc2016 /> O-H-LSD is formed by ] ]s, although the specific enzymes involved are unknown, and it does not appear to be known whether O-H-LSD is pharmacologically active or not.<ref name=Dol2015 /><ref name=Muc2016 /> The oral ] of LSD was crudely estimated as approximately 71% using previous data on ] administration of LSD.<ref name=Dol2015 /><ref name=Muc2016 /> The sample was equally divided between male and female subjects and there were no significant sex differences observed in the pharmacokinetics of LSD.<ref name=Dol2015 /><ref name=Muc2016 /> | |||
| ==Chemistry== | |||
| ] | |||
| LSD is a ] compound with two ]s at the ] atoms C-5 and C-8, so that theoretically four different ] of LSD could exist. LSD, also called (+)-''d''-LSD,<ref>{{Cite book |title=Handbook of Medical Hallucinogens |date=2021 |publisher=Guilford Publications |isbn=9781462545452 |pages=160 |language=en |chapter=LSD |chapter-url=https://mcb.berkeley.edu/labs2/presti/sites/mcb.berkeley.edu.labs2.presti/files/u3/2021%20LSD%20Chapter%20Panik%20Presti.pdf |access-date=March 14, 2024 |archive-date=March 14, 2024 |archive-url=https://web.archive.org/web/20240314163910/https://mcb.berkeley.edu/labs2/presti/sites/mcb.berkeley.edu.labs2.presti/files/u3/2021%20LSD%20Chapter%20Panik%20Presti.pdf |url-status=live }}</ref> has the ] (5''R'',8''R''). 5''S'' stereoisomers of lysergamides do not exist in nature and are not formed during the synthesis from ]-lysergic acid. ], the C-5 stereocenter could be analysed as having the same configuration of the alpha carbon of the naturally occurring amino acid L-], the precursor to all biosynthetic ergoline compounds. | |||
| However, LSD and iso-LSD, the two C-8 isomers, rapidly interconvert in the presence of ], as the alpha proton is acidic and can be ] and reprotonated. Non-psychoactive iso-LSD which has formed during the synthesis can be separated by ] and can be isomerized to LSD. | |||
| Pure salts of LSD are ], emitting small flashes of white light when shaken in the dark.<ref name="tihkal" /> LSD is strongly ] and will glow bluish-white under ]. | |||
| ===Synthesis=== | |||
| LSD is an ] derivative. It is commonly synthesized by reacting ] with an activated form of ]. Activating reagents include ]<ref name="synth1">{{cite journal |vauthors=Monte AP, Marona-Lewicka D, Kanthasamy A, Sanders-Bush E, Nichols DE |date=March 1995 |title=Stereoselective LSD-like activity in a series of d-lysergic acid amides of (R)- and (S)-2-aminoalkanes |journal=Journal of Medicinal Chemistry |volume=38 |issue=6 |pages=958–66 |pmid=7699712 |doi=10.1021/jm00006a015}}</ref> and ]s.<ref name="synth2">{{cite journal |vauthors=Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM |date=September 2002 |title=Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD) |journal=Journal of Medicinal Chemistry |volume=45 |issue=19 |pages=4344–9 |pmid=12213075 |doi=10.1021/jm020153s}}</ref> Lysergic acid is made by alkaline ] of lysergamides like ], a substance usually derived from the ] ] on ]. Lysergic acid can also be produced synthetically, although these processes are not used in clandestine manufacture due to their low yields and high complexity.<ref>{{cite journal |vauthors=Kornfeld EC, Fornefeld EJ, Kline GB, Mann MJ, Morrison DE, Jones RG, Woodward RB |title=The Total Synthesis of Lysergic Acid |journal=Journal of the American Chemical Society |volume=78 |issue=13 |pages=3087–3114 |year=1956 |doi=10.1021/ja01594a039|bibcode=1956JAChS..78.3087K }}</ref><ref>{{cite journal |vauthors=Inuki S, Oishi S, Fujii N, Ohno H |title=Total synthesis of (+/-)-lysergic acid, lysergol, and isolysergol by palladium-catalyzed domino cyclization of amino allenes bearing a bromoindolyl group |journal=Organic Letters |volume=10 |issue=22 |pages=5239–42 |date=November 2008 |pmid=18956869 |doi=10.1021/ol8022648 |url=https://figshare.com/articles/journal_contribution/2663242}}</ref> | |||
| Albert Hofmann synthesized LSD in the following manner: (1) hydrazinolysis of ergotamine into D- and L-isolysergic acid hydrazide, (2) seperation of the enantiomers with di-(''p''-touyl)-D-tartaric acid to get D-isolysergic acid hydrazide, (3) enantiomerization into D-lysergic acid hydrazide, (4) substitution with HNO<sub>2</sub> to D-lysergic acid azide and (5) finally substitution with diethylamine to form D-lysergic acid diethylamide.<ref>{{cite journal | last1 = Nichols | first1 = David E. | title = Dark classics in chemical neuroscience: lysergic acid diethylamide (LSD) | journal = ACS Chemical Neuroscience | volume = 9 | issue = 10 | pages = 2331–2343 | year = 2018 | doi = 10.1021/acschemneuro.8b00403 | url = https://shaunlacob.com/wp-content/uploads/2020/12/DC-LSD.pdf | access-date = 5 January 2025 }}</ref> | |||
| ====Research==== | |||
| The precursor for LSD, ], has been produced by ] ].<ref>{{cite web |author=((National University of Singapore, Yong Loo Lin School of Medicine)) |date=10 February 2022 |title=Harvesting baker's yeast for aging-related therapeutics |website=ScienceDaily |url=https://www.sciencedaily.com/releases/2022/02/220210154135.htm |access-date=2023-05-04 |archive-date=November 27, 2022 |archive-url=https://web.archive.org/web/20221127230250/https://www.sciencedaily.com/releases/2022/02/220210154135.htm |url-status=live }} '''Journal Reference:''' {{cite journal |vauthors=Wong G, Lim LR, Tan YQ, Go MK, Bell DJ, Freemont PS, Yew WS |title=Reconstituting the complete biosynthesis of D-lysergic acid in yeast |journal=Nature Communications |volume=13 |issue=1 |pages=712 |date=February 2022 |doi=10.1038/s41467-022-28386-6 |pmid=35132076 |pmc=8821704 |bibcode=2022NatCo..13..712W}}</ref> | |||
| ===Dosage=== | |||
| ]]] | |||
| A single dose of LSD is typically between 40 and 500 micrograms—an amount roughly equal to one-tenth the mass of a grain of sand. Threshold effects can be felt with as little as 25 micrograms of LSD.<ref name="greiner">{{cite journal |vauthors=Greiner T, Burch NR, Edelberg R |title=Psychopathology and psychophysiology of minimal LSD-25 dosage; a preliminary dosage-response spectrum |journal=A.M.A. Archives of Neurology and Psychiatry |volume=79 |issue=2 |pages=208–10 |date=February 1958 |pmid=13497365 |doi=10.1001/archneurpsyc.1958.02340020088016}}</ref><ref>{{cite journal |vauthors=Meyer MA |title=Neurologic complications of anthrax: a review of the literature |journal=Archives of Neurology |volume=60 |issue=4 |pages=483–8 |date=April 2003 |pmid=12707059 |doi=10.1001/archneur.60.4.483 |doi-access=free |place=Schweiz}}</ref> The practice of using sub-threshold doses is called ].<ref>{{cite journal |vauthors=Polito V, Stevenson RJ |title=A systematic study of microdosing psychedelics |journal=PLOS ONE |volume=14 |issue=2 |pages=e0211023 |date=2019-02-06 |pmid=30726251 |pmc=6364961 |doi=10.1371/journal.pone.0211023 |bibcode=2019PLoSO..1411023P |doi-access=free}}</ref> Dosages of LSD are measured in ] (μg), or millionths of a gram. | |||
| In the mid-1960s, the most important ] LSD manufacturer (]) distributed LSD at a standard concentration of 270 μg,<ref name="LSD Samples Analysis">{{cite web| vauthors=Hidalgo E| year=2009| title=LSD Samples Analysis| publisher=Erowid| url=http://www.erowid.org/chemicals/lsd/lsd_article3.shtml| access-date=February 8, 2010| url-status=live| archive-url=https://web.archive.org/web/20100213145552/http://www.erowid.org/chemicals/lsd/lsd_article3.shtml| archive-date=February 13, 2010}}</ref> while street samples of the 1970s contained 30 to 300 μg. By the 1980s, the amount had reduced to between 100 and 125 μg, dropping more in the 1990s to the 20–80 μg range,<ref name="henderson-glass">{{cite book |vauthors=Henderson LA, Glass WJ |title=LSD: Still with us after all these years |year=1994 |isbn=978-0-7879-4379-0 |publisher=Jossey-Bass |location=San Francisco}}</ref> and even more in the 2000s (decade).<ref name="LSD Samples Analysis" /><ref>{{cite web| author=Fire & Earth Erowid| date=Nov 2003| title=LSD Analysis – Do we know what's in street acid?| url=http://www.erowid.org/chemicals/lsd/lsd_article1.shtml| publisher=Erowid| access-date=February 8, 2010| archive-url=https://web.archive.org/web/20100126215446/http://www.erowid.org/chemicals/lsd/lsd_article1.shtml| archive-date=January 26, 2010| url-status=live}}</ref> | |||
| ===Reactivity and degradation=== | |||
| "LSD," writes the chemist ], "is an unusually fragile molecule ... As a salt, in water, cold, and free from air and light exposure, it is stable indefinitely."<ref name="tihkal" /> | |||
| LSD has two ] protons at the tertiary stereogenic C5 and C8 positions, rendering these centers prone to ]. The C8 proton is more labile due to the electron-withdrawing ] attachment, but the removal of the ] proton at the C5 position (which was once also an alpha proton of the parent molecule ]) is assisted by the inductively withdrawing nitrogen and pi electron delocalisation with the ] ring.{{Citation needed|date=May 2011}} | |||
| LSD also has ]-type reactivity because of the electron-donating effects of the indole ring. Because of this, ] destroys LSD molecules on contact; even though chlorinated tap water contains only a slight amount of chlorine, the small quantity of compound typical to an LSD solution will likely be eliminated when dissolved in tap water.<ref name="tihkal" /> The ] between the 8-position and the ], being conjugated with the indole ring, is susceptible to ] attacks by water or ], especially in the presence of UV or other kinds of light. LSD often converts to "lumi-LSD," which is inactive in human beings.<ref name="tihkal" /> | |||
| A controlled study was undertaken to determine the stability of LSD in pooled urine samples.<ref>{{cite journal | vauthors = Li Z, McNally AJ, Wang H, Salamone SJ | title = Stability study of LSD under various storage conditions | journal = Journal of Analytical Toxicology | volume = 22 | issue = 6 | pages = 520–5 | date = October 1998 | pmid = 9788528 | doi = 10.1093/jat/22.6.520 | doi-access = free }}</ref> | |||
| The concentrations of LSD in urine samples were followed over time at various temperatures, in different types of storage containers, at various exposures to different wavelengths of light, and at varying pH values. These studies demonstrated no significant loss in LSD concentration at 25 °C for up to four weeks. After four weeks of incubation, a 30% loss in LSD concentration at 37 °C and up to a 40% at 45 °C were observed. Urine fortified with LSD and stored in amber glass or nontransparent polyethylene containers showed no change in concentration under any light conditions. The stability of LSD in transparent containers under light was dependent on the distance between the light source and the samples, the wavelength of light, exposure time, and the intensity of light. After prolonged exposure to heat in alkaline pH conditions, 10 to 15% of the parent LSD epimerized to iso-LSD. Under acidic conditions, less than 5% of the LSD was converted to iso-LSD. It was also demonstrated that trace amounts of metal ions in the buffer or urine could catalyze the decomposition of LSD and that this process can be avoided by the addition of ]. | |||
| ===Detection=== | |||
| ] can be used to test for the presence of LSD in a sample, turning purple upon reaction.<ref name="LSDEMCDDA">{{cite web |url=https://www.emcdda.europa.eu/publications/drug-profiles/lsd_en |title=Lysergide (LSD) drug profile |website=] (EMCDDA) |access-date=15 May 2023 |url-status=live |archive-url=https://web.archive.org/web/20230202152854/https://www.emcdda.europa.eu/publications/drug-profiles/lsd_en |archive-date=2 February 2023}}</ref>]] | |||
| LSD can be detected in concentrations larger than approximately 10% in a sample using ] and ]. However, detecting LSD in human tissues is more challenging due to its active dosage being significantly lower (in ]) compared to most other drugs (in ]).<ref name="ReferenceA">{{Cite journal | vauthors = Appel JB, Whitehead WE, Freedman DX |date= July 1968 |title=Motivation and the behavioral effects of LSD |journal=Psychonomic Science |language=en |volume=12 |issue=7 |pages=305–306 |doi=10.3758/BF03331322 |s2cid=144527673 |issn=0033-3131|doi-access=free }}</ref> | |||
| LSD may be quantified in urine for drug testing programs, in plasma or serum to confirm poisoning in hospitalized victims, or in whole blood for forensic investigations. The parent drug and its major metabolite are unstable in biofluids when exposed to light, heat, or alkaline conditions, necessitating protection from light, low-temperature storage, and quick analysis to minimize losses.<ref>R. Baselt, ''Disposition of Toxic Drugs and Chemicals in Man'', 12th edition, Biomedical Publications, Foster City, CA, 2020, pp. 1197–1199.</ref> Maximum plasma concentrations are typically observed 1.4 to 1.5 hours after oral administration of 100 μg and 200 μg, respectively, with a plasma half-life of approximately 2.6 hours (ranging from 2.2 to 3.4 hours among test subjects).<ref>{{cite journal | vauthors = Dolder PC, Schmid Y, Steuer AE, Kraemer T, Rentsch KM, Hammann F, Liechti ME | title = Pharmacokinetics and Pharmacodynamics of Lysergic Acid Diethylamide in Healthy Subjects | journal = Clinical Pharmacokinetics | volume = 56 | issue = 10 | pages = 1219–1230 | date = October 2017 | pmid = 28197931 | doi = 10.1007/s40262-017-0513-9 | pmc = 5591798 }}</ref> | |||
| Due to its potency in microgram quantities, LSD is often not included in standard pre-employment urine or hair analyses.<ref name="ReferenceA"/><ref name="pmid36753839">{{cite journal |vauthors=Jiaming Z, Xin W, Jiali Z, Hang R, Yunli Z, Ping X |title=Concentrations of LSD, 2-oxo-3-hydroxy-LSD, and iso-LSD in hair segments of 18 drug abusers |journal=Forensic Science International |volume=344 |date=March 2023 |pmid=36753839 |doi=10.1016/j.forsciint.2023.111578| s2cid=256574276}}</ref> However, advanced ] methods can detect LSD in biological samples even after a single use.<ref name="pmid36753839"/> | |||
| ==History== | |||
| {{quote box| quote= ... affected by a remarkable restlessness, combined with a slight dizziness. At home I lay down and sank into a not unpleasant intoxicated-like condition, characterized by an extremely stimulated imagination. In a dreamlike state, with eyes closed (I found the daylight to be unpleasantly glaring), I perceived an uninterrupted stream of fantastic pictures, extraordinary shapes with intense, ] play of colors. After some two hours this condition faded away.|source= —Albert Hofmann, on his first experience with LSD<ref name="hofmann1980">{{cite book | vauthors = Hofmann A |url=http://www.psychedelic-library.org/child.htm |title=LSD—My Problem Child |access-date=April 19, 2010 |via=The Psychedelic Library |archive-url=https://web.archive.org/web/20171215043651/http://www.psychedelic-library.org/child.htm |archive-date=15 December 2017 |url-status=live |publisher=McGraw-Hill |date=1980 |isbn=0-07-029325-2}}</ref>{{rp|p=15}}|width=25em}} | |||
| {{Main|History of LSD}} | |||
| Swiss chemist ] first synthesized LSD in 1938 from ], a chemical derived from the ] of ], an ] found in ], a fungus that infects grain.<ref name="EU2018" /><ref name="NIH2018C">{{cite web |title=Commonly Abused Drugs Charts |url=https://www.drugabuse.gov/drugs-abuse/commonly-abused-drugs-charts#lsd |website=National Institute on Drug Abuse |access-date=14 July 2018 |date=2 July 2018 |url-status=live |archive-url=https://web.archive.org/web/20200301183029/https://www.drugabuse.gov/drugs-abuse/commonly-abused-drugs-charts#lsd |archive-date=March 1, 2020}}</ref> LSD was the 25th of various ] Hofmann synthesized from lysergic acid while trying to develop a new ], hence the alternate name LSD-25. Hofmann discovered its effects in humans in 1943, after unintentionally ingesting an unknown amount, possibly absorbing it through his skin.<ref name="Hofmann2009">{{Cite book |vauthors=Hofmann A |title=LSD, my problem child: reflections on sacred drugs, mysticism, and science |location=Santa Cruz, CA |publisher=Multidisciplinary Association for Psychedelic Studies |year=2009 |isbn=978-0-9798622-2-9 |edition=4th |oclc=610059315}}</ref><ref name="Lee1992">{{Cite book |vauthors=Lee MA, Shlain B |title=Acid dreams: the complete social history of LSD: the CIA, the Sixties, and beyond |date=1992 |publisher=Grove Weidenfeld |isbn=0-8021-3062-3 |location=New York |oclc=25281992}}</ref><ref name="Ettinger2017">{{cite book |vauthors=Ettinger RH |title=Psychopharmacology |date=2017 |publisher=Psychology Press |isbn=978-1-351-97870-5 |page=226 |language=en |url=https://books.google.com/books?id=XT4lDwAAQBAJ&pg=PA226 |access-date=September 27, 2021 |url-status=live |archive-url=https://web.archive.org/web/20210927002241/https://books.google.com/books?id=XT4lDwAAQBAJ&pg=PA226 |archive-date=September 27, 2021}}</ref> LSD was subject to exceptional interest within the field of ] in the 1950s and early 1960s, with ] distributing LSD to researchers under the trademark name Delysid in an attempt to find a marketable use for it.<ref name="Lee1992" /> During this period, LSD was controversially administered to hospitalised schizophrenic autistic children, with varying degrees of therapeutic success.<ref>{{cite journal | vauthors = Freedman AM, Ebin EV, Wilson EA | title = Autistic schizophrenic children. An experiment in the use of d-lysergic acid diethylamide (LSD-25) | journal = Archives of General Psychiatry | volume = 6 | issue = 3 | pages = 203–213 | date = March 1962 | pmid = 13894863 | doi = 10.1001/archpsyc.1962.01710210019003 }}</ref><ref>{{cite journal | vauthors = Simmons JQ, Leiken SJ, Lovaas OI, Schaeffer B, Perloff B | title = Modification of autistic behavior with LSD-25 | journal = The American Journal of Psychiatry | volume = 122 | issue = 11 | pages = 1201–1211 | date = May 1966 | pmid = 5325567 | doi = 10.1176/ajp.122.11.1201 }}</ref><ref>{{cite journal | vauthors = Sigafoos J, Green VA, Edrisinha C, Lancioni GE | title = Flashback to the 1960s: LSD in the treatment of autism | journal = Developmental Neurorehabilitation | volume = 10 | issue = 1 | pages = 75–81 | date = 2007 | pmid = 17608329 | doi = 10.1080/13638490601106277 }}</ref><ref>{{cite journal | vauthors = Abramson HA | title = The use of LSD (d-lysergic acid diethylamide) in the therapy of children (a brief review) | journal = The Journal of Asthma Research | volume = 5 | issue = 2 | pages = 139–143 | date = December 1967 | pmid = 4865578 | doi = 10.3109/02770906709104325 }}</ref> | |||
| LSD-assisted ] was used in the 1950s and early 1960s by psychiatrists such as ], who pioneered the application of LSD to the treatment of ], with promising results.<ref name="Lee1992"/><ref>{{Cite web |title=Psychiatric Research with Hallucinogens |website=www.druglibrary.org |url=https://www.druglibrary.org/schaffer/lsd/grob.htm |access-date=2021-07-26|archive-date=July 26, 2021|archive-url=https://web.archive.org/web/20210726203733/https://www.druglibrary.org/schaffer/lsd/grob.htm |url-status=live}}</ref><ref name="Use of d-lysergic acid diethylamide">{{cite journal |vauthors=Chwelos N, Blewett DB, Smith CM, Hoffer A |title=Use of d-lysergic acid diethylamide in the treatment of alcoholism |journal=Quarterly Journal of Studies on Alcohol |volume=20 |issue=3 |pages=577–590 |date=September 1959 |pmid=13810249 |doi=10.15288/qjsa.1959.20.577}}</ref><ref name="Lysergic acid diethylamide LSD fo">{{cite journal |vauthors=Krebs TS, Johansen PØ |title=Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials |journal=Journal of Psychopharmacology |volume=26 |issue=7 |pages=994–1002 |date=July 2012 |pmid=22406913 |doi=10.1177/0269881112439253 |s2cid=10677273}}</ref> Osmond coined the term "psychedelic" (lit. ''mind manifesting'') as a term for LSD and related ]s, superseding the previously held "]" model in which LSD was believed to mimic ]. In contrast to schizophrenia, LSD can induce ] experiences, or mental states that transcend the experience of everyday consciousness, with lasting psychological benefit.<ref name="pmid26841800" /><ref name="Lee1992" /> During this time, the ] (CIA) began using LSD in the research project ], which used ] to aid ]. The CIA administered LSD to unwitting test subjects to observe how they would react, the most well-known example of this being ].<ref name="Lee1992" /> LSD was one of several psychoactive substances evaluated by the ] as possible non-lethal incapacitants in the ].<ref name="Lee1992" /> | |||
| In the 1960s, LSD and other psychedelics were adopted by and became synonymous with, the ] due to their perceived ability to expand consciousness. This resulted in LSD being viewed as a cultural threat to American values and the ], and it was designated as a ] (illegal for medical as well as recreational use) substance in 1968.<ref>{{Cite book |author=United States Congress House Committee on Interstate and Foreign Commerce Subcommittee on Public Health and Welfare |url=https://books.google.com/books?id=qbY6xQEACAAJ |title=Increased Controls Over Hallucinogens and Other Dangerous Drugs |date=1968 |publisher=U.S. Government Printing Office |access-date=August 3, 2021|archive-date=July 13, 2020 |archive-url=https://web.archive.org/web/20200713014802/https://books.google.com/books?id=qbY6xQEACAAJ|url-status=live}}</ref> It was listed as a ] by the ] in 1971 and currently has no approved medical uses.<ref name="EU2018" /> {{As of|2017}}, about 10% of people in the United States have used LSD at some point in their lives, while 0.7% have used it in the last year.<ref name="NIH2018B">{{cite web|author=National Institute on Drug Abuse|title=Hallucinogens |url=https://www.drugabuse.gov/drugs-abuse/hallucinogens |access-date=14 July 2018|archive-date=June 3, 2020|archive-url=https://web.archive.org/web/20200603125635/https://www.drugabuse.gov/drugs-abuse/hallucinogens|url-status=live}}</ref> It was most popular in the 1960s to 1980s.<ref name="EU2018" /> The use of LSD among US adults increased by 56.4% from 2015 to 2018.<ref>{{cite journal |vauthors=Yockey RA, Vidourek RA, King KA |title=Trends in LSD use among US adults: 2015–2018 |journal=Drug and Alcohol Dependence |volume=212 |pages=108071 |date=July 2020 |pmid=32450479 |doi=10.1016/j.drugalcdep.2020.108071 |s2cid=218893155}}</ref> | |||
| LSD was first synthesized on November 16, 1938<ref>{{cite journal |vauthors=Hofmann A |author-link=Albert Hofmann| translator=Ott J |title=LSD Ganz Persönlich |trans-title=LSD: Completely Personal |date=Summer 1969 |url=http://www.maps.org/news-letters/v06n3/06346hof.html |journal=MAPS |volume=6 |issue=69 |language=de |archive-url=https://web.archive.org/web/20131206032629/http://www.maps.org/news-letters/v06n3/06346hof.html |archive-date=6 December 2013}}</ref> by Swiss chemist ] at the ] Laboratories in ], Switzerland as part of a large research program searching for medically useful ] derivatives. The abbreviation "LSD" is from the German "Lysergsäurediethylamid".<ref>{{cite book |url=https://books.google.com/books?id=TNXeDAAAQBAJ&pg=PT342 |title=Medicinal Chemistry: A Molecular and Biochemical Approach |vauthors=Nogrady T, Weaver DF |date=2005 |publisher=Oxford University Press |isbn=978-0-19-028296-7 |page=342 |language=en |access-date=March 14, 2020 |url-status=live |archive-url=https://web.archive.org/web/20210308133740/https://www.google.com/books/edition/Medicinal_Chemistry/TNXeDAAAQBAJ?hl=en&gbpv=1&dq=%22LysergS%C3%A4ureDiethylamid%22+abreviation+LSD&pg=PT342 |archive-date=March 8, 2021}}</ref> | |||
| ] in 2006]] | |||
| LSD's ] properties were discovered 5 years later when Hofmann himself accidentally ingested an unknown quantity of the chemical.<ref name="hyponichols">{{cite web |title=Hypothesis on Albert Hofmann's Famous 1943 "Bicycle Day" |url=http://www.erowid.org/general/conferences/conference_mindstates4_nichols.shtml |access-date=September 27, 2007 |vauthors=Nichols D |date=May 24, 2003 |website=Hofmann Foundation |archive-date=September 22, 2007 |archive-url=https://web.archive.org/web/20070922215008/http://www.erowid.org/general/conferences/conference_mindstates4_nichols.shtml |url-status=live}}</ref> The first intentional ingestion of LSD occurred on April 19, 1943,<ref name="hofmann1980" /> when Hofmann ingested 250 ] of LSD. He said this would be a threshold dose based on the dosages of other ergot alkaloids. Hofmann found the effects to be much stronger than he anticipated.<ref name="histlsd">{{cite web |url=http://www.a1b2c3.com/drugs/lsd01.htm|title=History Of LSD |access-date=September 27, 2007 |vauthors=Hofmann A |archive-url=https://web.archive.org/web/20070904212518/http://www.a1b2c3.com/drugs/lsd01.htm |archive-date=September 4, 2007}}</ref> Sandoz Laboratories introduced LSD as a psychiatric drug in 1947 and marketed LSD as a psychiatric panacea, hailing it "as a cure for everything from schizophrenia to criminal behavior, 'sexual perversions', and alcoholism."<ref name="LSD: The Drug">{{cite report |date=Oct 1995 |chapter=LSD: The Drug |title=LSD in the United States |publisher=U.S. Department of Justice, Drug Enforcement Administration |chapter-url=http://www.usdoj.gov/dea/pubs/lsd/lsd-4.htm |access-date=November 27, 2010 |archive-date=April 27, 1999 |archive-url=https://web.archive.org/web/19990427145322/http://www.usdoj.gov/dea/pubs/lsd/lsd-4.htm}}</ref> Sandoz would send the drug for free to researchers investigating its effects.<ref name="Hofmann2009"/> | |||
| ] | |||
| Beginning in the 1950s, the US ] (CIA) began a research program code-named ]. The CIA introduced LSD to the United States, purchasing the entire world's supply for $240,000 and propagating the LSD through CIA ] to American hospitals, clinics, prisons, and research centers.<ref>{{cite news |title=The CIA's Secret Quest For Mind Control: Torture, LSD And A 'Poisoner In Chief' |url=https://www.npr.org/2019/09/09/758989641/the-cias-secret-quest-for-mind-control-torture-lsd-and-a-poisoner-in-chief |website=NPR.org |access-date=6 October 2019 |language=en |archive-date=June 28, 2021 |archive-url=https://web.archive.org/web/20210628081520/https://www.npr.org/2019/09/09/758989641/the-cias-secret-quest-for-mind-control-torture-lsd-and-a-poisoner-in-chief |url-status=live }}</ref> Experiments included administering LSD to CIA employees, military personnel, doctors, other government agents, prostitutes, mentally ill patients, and members of the general public to study their reactions, usually without the subjects' knowledge. The project was revealed in the US congressional ] in 1975. | |||
| In 1963, the Sandoz patents on LSD expired<ref name="henderson-glass"/> and the Czech company Spofa began to produce the substance.<ref name="Hofmann2009" /> Sandoz stopped the production and distribution in 1965.<ref name="Hofmann2009" /> | |||
| Several figures, including ], ], and ], had begun to advocate the consumption of LSD. LSD became central to the counterculture of the 1960s.<ref>{{cite web |url=http://www.druglibrary.org/schaffer/Library/studies/cu/CU50.html |title=How LSD was popularized |vauthors=Brecher EM |collaboration=Editors of Consumer Reports Magazine |date=1972 |publisher=Druglibrary.org |access-date=20 June 2012 |url-status=live |archive-url=https://web.archive.org/web/20120513233708/http://druglibrary.org/schaffer/Library/studies/cu/CU50.html |archive-date= 13 May 2012}}</ref> In the early 1960s the use of LSD and other hallucinogens was advocated by new proponents of consciousness expansion such as Leary, Huxley, ] and ],<ref>{{cite web |vauthors=Applebaum A |author-link=Anne Applebaum |url=http://www.huffingtonpost.com/2010/01/26/did-the-death-of-communis_n_435939.html |title=Did The Death Of Communism Take Koestler And Other Literary Figures With It? |work=] |date=26 January 2010 |archive-url=http://archive.wikiwix.com/cache/20110714161713/http://www.huffingtonpost.com/2010/01/26/did-the-death-of-communis_n_435939.html |archive-date=14 July 2011}}</ref><ref>{{cite web |title=''Out-Of-Sight!'' SMiLE Timeline |url=http://pages.cthome.net/tobelman/The_Out-Of-Sight_SMiLE_Site.html|access-date=October 30, 2011|archive-url=https://web.archive.org/web/20100201234435/http://pages.cthome.net/tobelman/The_Out-Of-Sight_SMiLE_Site.html |archive-date=February 1, 2010}}</ref> and according to L. R. Veysey they profoundly influenced the thinking of the new generation of youth.<ref>{{cite book |vauthors=Veysey LR |title=] |location=Chicago IL |publisher=University of Chicago Press |date=1978 |isbn=0-226-85458-2 |page=437}}</ref> | |||
| On October 24, 1968, possession of LSD was made illegal in the United States.<ref>{{cite web |url=http://www.erowid.org/psychoactives/law/law_fed_staggers-dodd.pdf |author=United States Congress |title=Staggers-Dodd Bill, Public Law 90-639 |date=October 24, 1968 |access-date=September 8, 2009 |url-status=live |archive-url=https://web.archive.org/web/20100509025200/http://www.erowid.org/psychoactives/law/law_fed_staggers-dodd.pdf |archive-date=May 9, 2010}}</ref> The last ] approved study of LSD in patients ended in 1980, while a study in healthy volunteers was made in the late 1980s. Legally approved and regulated psychiatric use of LSD continued in Switzerland until 1993.<ref>{{cite web |url=http://www.maps.org/news-letters/v05n3/05303psy.html |vauthors=Gasser P |title=Psycholytic Therapy with MDMA and LSD in Switzerland |year=1994 |access-date=September 8, 2009 |url-status=live | archive-date=October 11, 2009 |archive-url=https://web.archive.org/web/20091011083518/http://www.maps.org/news-letters/v05n3/05303psy.html}}</ref> | |||
| In November 2020, Oregon became the first US state to decriminalize possession of small amounts of LSD after voters approved ].<ref>{{Cite web|title=Oregon becomes first state to legalize magic mushrooms as more states ease drug laws in 'psychedelic renaissance'|vauthors=Feuer W|date=November 4, 2020 |url=https://www.cnbc.com/2020/11/04/oregon-becomes-first-state-to-legalize-magic-mushrooms-as-more-states-ease-drug-laws.html |website=CNBC|access-date=November 7, 2020 |url-status=live|archive-date=November 4, 2020|archive-url=https://web.archive.org/web/20201104155737/https://www.cnbc.com/2020/11/04/oregon-becomes-first-state-to-legalize-magic-mushrooms-as-more-states-ease-drug-laws.html}}</ref> | |||
| ==Society and culture== | |||
| ===Counterculture=== | |||
| By the mid-1960s, the youth ]s in California, particularly in San Francisco, had widely adopted the use of hallucinogenic drugs, including LSD. The first major underground LSD factory was established by ].<ref name=DeRogatispp8-9>{{cite book |vauthors=DeRogatis J |title=Turn On Your Mind: Four Decades of Great Psychedelic Rock |location=Milwaukie, Michigan |publisher=Hal Leonard |date=2003 |isbn=0-634-05548-8 |pages=8–9}}</ref> Around this time, the ], associated with novelist ], organized the ], events in San Francisco involving LSD consumption, accompanied by light shows and improvised music.<ref name=pc41>{{cite web |url=https://digital.library.unt.edu/ark:/67531/metadc19800/m1/ |title=Show 41 – The Acid Test: Psychedelics and a sub-culture emerge in San Francisco. ] |publisher=Digital.library.unt.edu |format=audio |access-date=May 6, 2011 |url-status=live |archive-url=http://archive.wikiwix.com/cache/20110629170356/https://digital.library.unt.edu/ark:/67531/metadc19800/m1/ |archive-date=June 29, 2011}}</ref><ref name=Hicks2000p60>{{cite book | vauthors = Hicks M | title = Sixties Rock: Garage, Psychedelic, and Other Satisfactions Music in American Life | location = Chicago, IL | publisher = University of Illinois Press | date = 2000 | isbn = 0-252-06915-3 | page = 60 }}</ref> Their activities, including cross-country trips in a psychedelically decorated bus and interactions with major figures of the beat movement, were later documented in ]'s '']'' (1968).<ref name=Mann2009p87>{{cite book | vauthors = Mann J | title = Turn on and Tune in: Psychedelics, Narcotics and Euphoriants | publisher = Royal Society of Chemistry | date = 2009 | isbn = 978-1-84755-909-8 | page = 87 }}</ref> | |||
| In San Francisco's Haight-Ashbury neighborhood, the Psychedelic Shop was opened in January 1966 by brothers Ron and Jay Thelin to promote the safe use of LSD. This shop played a significant role in popularizing LSD in the area and establishing ] as the epicenter of the hippie counterculture. The Thelins also organized the ] in Golden Gate Park in October 1966, protesting against California's ban on LSD.<ref>{{Cite web|title=OBITUARY — Ron Thelin |url=https://www.sfgate.com/news/article/OBITUARY-Ron-Thelin-2989153.php |vauthors=Taylor M |date=1996-03-22 |website=SFGate |access-date=2020-05-13 |url-status=live |archive-date=August 28, 2021 |archive-url=https://web.archive.org/web/20210828153344/https://www.sfgate.com/news/article/OBITUARY-Ron-Thelin-2989153.php}}</ref><ref>{{cite journal |vauthors=Davis JC |title=The business of getting high: head shops, countercultural capitalism, and the marijuana legalization movement. |journal=The Sixties |date=January 2015 |volume=8 |issue=1 |pages=27–49 |doi=10.1080/17541328.2015.1058480 |hdl=11603/7422 |s2cid=142795620 |hdl-access=free}}</ref> | |||
| A similar movement developed in London, led by British academic ], who first tried LSD in America in 1961. After experiencing LSD and interacting with notable figures such as ], ], and ], Hollingshead played a key role in the famous LSD research at Millbrook before moving to New York City for his experiments. In 1965, he returned to the UK and founded the World Psychedelic Center in Chelsea, London.<ref>{{cite book | vauthors = Conners P |title=White Hand Society - The Psychedelic Partnership of Timothy Leary and Allen Ginsberg |publisher=City Lights Books |year=2010 |isbn=9780872865358 |page= |url-access=registration |url=https://archive.org/details/isbn_9780872865358/page/148 }}</ref> | |||
| ===Music and Art=== | |||
| ] newspaper '']'', 1967]] | |||
| The influence of LSD in the realms of music and art became pronounced in the 1960s, especially through the Acid Tests and related events involving bands like the ], ], and ]. San Francisco-based artists such as ], ], and ] contributed to this movement through their psychedelic poster and album art. ], in particular, became central to the culture of "Deadheads," with their music heavily influenced by LSD.<ref name=Jarnow/> | |||
| In the United Kingdom, Michael Hollingshead, reputed for introducing LSD to various artists and musicians like ], ], ], and members of ], played a significant role in the drug's proliferation in the British art and music scene. Despite LSD's illegal status from 1966, it was widely used by groups including ], ], and ]. Their experiences influenced works such as the Beatles' '']'' and Cream's '']'', featuring psychedelic-themed music and artwork.<ref>{{cite magazine |vauthors=Gilmore M |url=https://www.rollingstone.com/feature/beatles-acid-test-how-lsd-opened-the-door-to-revolver-251417/ |title=Beatles' Acid Test: How LSD Opened the Door to 'Revolver' |magazine=Rolling Stone |date=August 25, 2016 |access-date=December 9, 2021 |archive-date=December 3, 2020 |archive-url=https://web.archive.org/web/20201203211257/https://www.rollingstone.com/feature/beatles-acid-test-how-lsd-opened-the-door-to-revolver-251417/ |url-status=live}}</ref> | |||
| Psychedelic music of the 1960s often sought to replicate the LSD experience, incorporating exotic instrumentation, electric guitars with effects pedals, and elaborate studio techniques. Artists and bands utilized instruments like sitars and tablas, and employed studio effects such as backward tapes, panning, and phasing.<ref>{{cite book |vauthors=Rubin R, Melnick JP |title=Immigration and American Popular Culture: an Introduction |location=New York, NY |publisher=New York University Press |date=2007 |isbn=978-0-8147-7552-3 |pages=162–4}}</ref><ref>{{cite book |vauthors=Prown P, Newquist HP, Eiche JF |title=Legends of Rock Guitar: the Essential Reference of Rock's Greatest Guitarists |location=London |publisher=Hal Leonard Corporation, 1997 |isbn=0-7935-4042-9 |pages=48 |year=1997}}</ref> Songs such as ]'s "Illegal Smile" and the Beatles' "]" have been associated with LSD, although the latter's authors denied such claims.<ref name=Sheff>{{cite book |vauthors=Sheff D |year=2000 |author-link=David Sheff |title=All We Are Saying: The Last Major Interview with John Lennon and Yoko Ono |publisher=St. Martin's Press |location=New York |isbn=978-0-312-25464-3 |url=https://archive.org/details/allwearesayingla00lenn |url-access=registration}}</ref>{{page needed|date=January 2024}}<ref name="life">{{cite magazine |vauthors=Thompson T |date=16 June 1967 |title=The New Far-Out Beatles |magazine=Life |url=https://books.google.com/books?id=lVYEAAAAMBAJ&pg=101 |location=Chicago |publisher=Time Inc. |pages=101 |access-date=8 Dec 2016 |archive-date=November 17, 2021 |archive-url=https://web.archive.org/web/20211117042255/https://books.google.com/books?id=lVYEAAAAMBAJ&pg=101 |url-status=live}}</ref> | |||
| Contemporary artists influenced by LSD include ] in the visual arts,<ref>{{cite book |title=Keith Haring: Journey of the Radiant Baby |url=https://books.google.com/books?id=PElY27UXXkYC |publisher=Bunker Hill Publishing |year=2006 |isbn=1593730527 |vauthors=Haring K |page=25 |access-date=December 5, 2023 |archive-date=October 2, 2023 |archive-url=https://web.archive.org/web/20231002134101/https://books.google.com/books?id=PElY27UXXkYC |url-status=live }}</ref> various ] creators,<ref>{{cite news |url=https://www.vice.com/en/article/newv7g/why-drugs-genres-match-mdma-raves-shrooms-psychedelia-rap-lean |publisher=Vice |author=Daisy Jones |date=5 June 2017 |title=Why Certain Drugs Make Specific Genres Sound So Good |access-date=December 5, 2023 |archive-date=December 5, 2023 |archive-url=https://web.archive.org/web/20231205200458/https://www.vice.com/en/article/newv7g/why-drugs-genres-match-mdma-raves-shrooms-psychedelia-rap-lean |url-status=live }}</ref> and the ] ].<ref>{{cite web |url=https://liveforlivemusic.com/features/phish-matisyahu-nyc/ |title=Phishin' With Matisyahu: How LSD "Turned My Entire World Inside Out" |author=Kendall Deflin |date=22 June 2017 |access-date=December 5, 2023 |archive-date=September 30, 2023 |archive-url=https://web.archive.org/web/20230930095840/https://liveforlivemusic.com/features/phish-matisyahu-nyc/ |url-status=live }}</ref> The 2018 ] play '']'' is inspired by the author's interest in the history of LSD.<ref>{{Cite web |title=How LSD influenced Western culture |url=https://www.bbc.com/culture/article/20181016-how-lsd-influenced-western-culture |access-date=2024-01-08 |website=www.bbc.com |archive-date=November 27, 2020 |archive-url=https://web.archive.org/web/20201127003729/https://www.bbc.com/culture/article/20181016-how-lsd-influenced-western-culture |url-status=live }}</ref> | |||
| ===Legal status=== | |||
| The ] ] of 1971 mandates that signing parties, including the United States, Australia, New Zealand, and most of Europe, prohibit LSD. Enforcement of these laws varies by country. The convention allows medical and scientific research with LSD.<ref>{{cite web |work=UN Convention on Psychotropic Substances |date=1971 |url=http://www.unodc.org/pdf/convention_1971_en.pdf |title=Final act of the United Nations Conference |archive-url=https://web.archive.org/web/20120415011336/http://www.unodc.org/pdf/convention_1971_en.pdf |archive-date=15 April 2012}}</ref> | |||
| ====Australia==== | |||
| In Australia, LSD is classified as a Schedule 9 prohibited substance under the Poisons Standard (February 2017), indicating it may be abused or misused and its manufacture, possession, sale, or use should be prohibited except for approved research purposes.<ref name="Poisons Standard">{{cite web |url=https://www.legislation.gov.au/Details/F2017L00057 |title=Poisons Standard |date=July 2016 |archive-url=https://web.archive.org/web/20170302025331/https://www.legislation.gov.au/Details/F2017L00057 |archive-date=2 March 2017 |work=Therapeutic Goods Administration |publisher=Australian Government Department of Health}}</ref> In Western Australia, the Misuse of Drugs Act 1981 provides guidelines for possession and trafficking of substances like LSD.<ref>{{cite web |title=Misuse of Drugs Act 1981 |date=18 November 2015 |url=http://www.slp.wa.gov.au/pco/prod/FileStore.nsf/Documents/MRDocument:28280P/$FILE/Misuse%20Of%20Drugs%20Act%201981%20-%20%5B06-e0-00%5D.pdf?OpenElement |publisher=Government of Western Australia |archive-url=https://web.archive.org/web/20151222180141/http://www.slp.wa.gov.au/pco/prod/FileStore.nsf/Documents/MRDocument%3A28280P/%24FILE/Misuse%20Of%20Drugs%20Act%201981%20-%20%5B06-e0-00%5D.pdf?OpenElement |archive-date=22 December 2015}}</ref> | |||
| ====Canada==== | |||
| In Canada, LSD is listed under Schedule III of the Controlled Drugs and Substances Act. Unauthorized possession and trafficking of the substance can lead to significant legal penalties.<ref name="cdasa">{{cite web|url=http://laws-lois.justice.gc.ca/eng/acts/C-38.8/page-26.html#h-30 |title=Controlled Drugs and Substances Act |access-date=December 15, 2013 |publisher=Canadian Department of Justice |year=1996 |author=Canadian government |website=Justice Laws |archive-url=https://web.archive.org/web/20131215170432/http://laws-lois.justice.gc.ca/eng/acts/C-38.8/page-26.html |archive-date=December 15, 2013 }}</ref> | |||
| ====United Kingdom==== | |||
| In the United Kingdom, LSD is a Class A drug under the Misuse of Drugs Act 1971, making unauthorized possession and trafficking punishable by severe penalties. The Runciman Report and Transform Drug Policy Foundation have made recommendations and proposals regarding the legal regulation of LSD and other psychedelics.<ref>{{cite web |url=http://www.druglibrary.org/schaffer/Library/studies/runciman/pf3.htm |title=Drugs and the law: Report of the inquiry into the Misuse of Drugs Act 1971 |archive-url=https://web.archive.org/web/20160130144204/http://www.druglibrary.org/schaffer/Library/studies/runciman/pf3.htm |archive-date=30 January 2016 |location=London |publisher=Police Foundation |date=2000 |work=]}}</ref><ref>{{cite web |url=http://www.tdpf.org.uk/blueprint%20download.htm |title=After the War on Drugs: Blueprint for Regulation |archive-url=https://web.archive.org/web/20131005145241/http://www.tdpf.org.uk/blueprint%20download.htm |archive-date=5 October 2013 |work=Transform Drug Policy Foundation |date=2009}}</ref> | |||
| ====United States==== | |||
| In the United States, LSD is classified as a Schedule I controlled substance under the Controlled Substances Act of 1970, making its manufacture, possession, and distribution illegal without a DEA license. The law considers LSD to have a high potential for abuse, no legitimate medical use, and to be unsafe even under medical supervision. The US Supreme Court case Neal v. United States (1995) clarified the sentencing guidelines related to LSD possession.<ref>{{cite court| litigants=Neal v. United States| reporter=U.S.| vol=516| opinion=284| pinpoint=| court=| year=1996| url=https://www.law.cornell.edu/supct/html/94-9088.ZO.html| archive-url=https://web.archive.org/web/20160409213251/https://www.law.cornell.edu/supct/html/94-9088.ZO.html| url-status=live}}, originating from U.S. v. Neal, 46 F.3d 1405 (7th Cir. 1995)</ref> | |||
| Oregon decriminalized personal possession of small amounts of drugs, including LSD, in February 2021, and California has seen legislative efforts to decriminalize psychedelics.<ref>{{Cite web|vauthors=Jaeger K|date=2021-06-29|title=California Lawmakers Approve Bill To Legalize Psychedelics Possession In Committee|url=https://www.marijuanamoment.net/california-lawmakers-approve-bill-to-legalize-psychedelics-possession-in-committee/|access-date=2021-07-08|website=Marijuana Moment|language=en-US|archive-date=July 9, 2021|archive-url=https://web.archive.org/web/20210709172122/https://www.marijuanamoment.net/california-lawmakers-approve-bill-to-legalize-psychedelics-possession-in-committee/|url-status=live}}</ref> | |||
| ====Mexico==== | |||
| Mexico decriminalized the possession of small amounts of drugs, including LSD, for personal use in 2009. The law specifies possession limits and establishes that possession is not a crime within designated quantities.<ref>{{cite web |url=http://www.elpensador.com.mx/2009/10/17/Ley-de-Narcomenudeo/ |title=Ley de Narcomenudeo |archive-url=https://web.archive.org/web/20101130224127/http://www.elpensador.com.mx/2009/10/17/Ley-de-Narcomenudeo/ |archive-date=30 November 2010 |language=es |work=El Pensador |date=17 October 2009}}</ref> | |||
| ====Czech Republic==== | |||
| In the Czech Republic, possession of "amount larger than small" of LSD is criminalized, while possession of smaller amounts is a misdemeanor. The definition of "amount larger than small" is determined by judicial practice and specific regulations.<ref name="Cz expl rep">{{cite report |publisher=Parliament of the Czech Republic |year=1998 |title=Explanatory Report to Act No. 112/1998 Coll., which amends the Act No. 140/1961 Coll., the Criminal Code, and the Act No. 200/1990 Coll., on misdemeanors |location=Prague |language=cs}}</ref><ref>{{Citation |author=Supreme Court of the Czech Republic |author-link=Supreme Court of the Czech Republic |date=25 February 2012 |title=6 Tdo 156/2010 }}</ref> | |||
| ===Economics=== | |||
| ====Production==== | |||
| ] | |||
| An active dose of LSD is very minute, allowing a large number of doses to be synthesized from a comparatively small amount of raw material. Twenty-five kilograms of precursor ] ] can produce 5–6 kg of pure crystalline LSD; this corresponds to around 50–60 million doses at 100 μg. Because the masses involved are so small, concealing and transporting illicit LSD is much easier than smuggling ], ], or other illegal drugs.<ref name="DEA-pub">{{cite web|author=DEA |year=2007 |title=LSD Manufacture – Illegal LSD Production |url=https://fas.org/irp/agency/doj/dea/product/lsd/lsd-5.htm |website=LSD in the United States |publisher=U.S. Department of Justice Drug Enforcement Administration |archive-url=https://web.archive.org/web/20070829023659/https://fas.org/irp/agency/doj/dea/product/lsd/lsd-5.htm|archive-date=August 29, 2007}}{{Dead link|date=January 2015}}</ref> | |||
| Manufacturing LSD requires laboratory equipment and experience in the field of ]. It takes two to three days to produce 30 to 100 grams of pure compound. It is believed that LSD is not usually produced in large quantities, but rather in a series of small batches. This technique minimizes the loss of precursor chemicals in case a step does not work as expected.<ref name="DEA-pub"/> | |||
| =====Forms===== | |||
| ] | |||
| LSD is produced in crystalline form and is then mixed with ]s or redissolved for production in ingestible forms. Liquid solution is either distributed in small vials or, more commonly, sprayed onto or soaked into a distribution medium. Historically, LSD solutions were first sold on sugar cubes, but practical considerations{{clarification needed|date=September 2024}} forced a change to ] form. Appearing in 1968 as an orange tablet measuring about 6 mm across, "Orange Sunshine" acid was the first largely available form of LSD after its possession was made illegal. ], a prominent chemist, made some of these tablets, but said that most "Sunshine" in the USA came by way of Ronald Stark, who imported approximately thirty-five million doses from Europe.<ref name=Stafford1992>{{cite book |vauthors=Stafford P |year=1992 |title=Psychedelics Encyclopaedia |chapter=Chapter 1 – The LSD Family |pages=62 |edition=3rd |publisher=Ronin Publishing |isbn=978-0-914171-51-5}}</ref> | |||
| Over some time, tablet dimensions, weight, shape and concentration of LSD evolved from large (4.5–8.1 mm diameter), heavyweight (≥150 mg), round, high concentration (90–350 μg/tab) dosage units to small (2.0–3.5 mm diameter) lightweight (as low as 4.7 mg/tab), variously shaped, lower concentration (12–85 μg/tab, average range 30–40 μg/tab) dosage units. LSD tablet shapes have included cylinders, cones, stars, spacecraft, and heart shapes. The smallest tablets became known as "Microdots."<ref name=Laing2003>{{cite book |vauthors=Laing RR, Beyerstein BL, Siegel JA |year=2003 |title=Hallucinogens: A Forensic Drug Handbook |chapter=Chapter 2.2 – Forms of the Drug |chapter-url=https://books.google.com/books?id=l1DrqgobbcwC |pages=39–41 |publisher=Academic Press |isbn=978-0-12-433951-4 |access-date=May 12, 2020 |archive-date=February 2, 2021 |archive-url=https://web.archive.org/web/20210202134552/https://books.google.com/books?id=l1DrqgobbcwC |url-status=live}}</ref> | |||
| After tablets came "computer acid" or "blotter paper LSD," typically made by dipping a preprinted sheet of ] into an LSD/water/alcohol solution.<ref name=Stafford1992/><ref name=Laing2003/> More than 200 types of LSD tablets have been encountered since 1969 and more than 350 blotter paper designs have been observed since 1975.<ref name=Laing2003/> About the same time as blotter paper LSD came "Windowpane" (AKA "Clearlight"), which contained LSD inside a thin ] square a quarter of an inch (6 mm) across.<ref name=Stafford1992/> <!-- Please do not add any street names here unless you can provide evidence for their notability and importance! Additions not referenced to a reliable source will be removed immediately. The goal of an encyclopedia is to provide a "ready reference" of key concepts, not give an exhaustive list of every detail.--> LSD has been sold under a wide variety of often short-lived and regionally restricted street names including Acid, Trips, Uncle Sid, Blotter, ], Alice and doses, as well as names that reflect the designs on the sheets of blotter paper.<ref name="erowid-faq"/><ref>{{cite web| title=Street Terms: Drugs and the Drug Trade| date=April 5, 2005| url=http://www.whitehousedrugpolicy.gov/streetterms/ByType.asp?intTypeID=6| publisher=]| access-date=January 31, 2007| url-status=live| archive-url=https://web.archive.org/web/20090418031446/http://www.whitehousedrugpolicy.gov/streetTerms/ByType.asp?intTypeID=6| archive-date=April 18, 2009}}</ref> Authorities have encountered the drug in other forms—including powder or crystal, and capsule.<ref>{{cite web |author=DEA |year=2008 |title=Photo Library (page 2) |publisher=US Drug Enforcement Administration |url=http://www.usdoj.gov/dea/photo_library2.html#lsd |access-date=June 27, 2008 |archive-url=https://web.archive.org/web/20080623111640/http://www.usdoj.gov/dea/photo_library2.html |archive-date=June 23, 2008}}</ref> | |||
| =====Modern distribution===== | |||
| LSD manufacturers and traffickers in the United States can be categorized into two groups: A few large-scale producers, and an equally limited number of small, clandestine chemists, consisting of independent producers who, operating on a comparatively limited scale, can be found throughout the country.<ref>{{cite book |vauthors=MacLean JR, Macdonald DC, Ogden F, Wilby E |chapter=LSD-25 and mescaline as therapeutic adjuvants. |veditors=Abramson H |title=The Use of LSD in Psychotherapy and Alcoholism |publisher=Bobbs-Merrill |location=New York |date=1967 |pages=407–426}}</ref><ref>{{cite book |vauthors=Ditman KS, Bailey JJ |chapter=Evaluating LSD as a psychotherapeutic agent |veditors=Hoffer A |title=A program for the treatment of alcoholism: LSD, malvaria, and nicotinic acid |pages=353–402}}</ref> | |||
| As a group, independent producers are of less concern to the ] than the large-scale groups because their product reaches only local markets.<ref name="LSD: The Drug"/> | |||
| Many LSD dealers and chemists describe a religious or humanitarian purpose that motivates their illicit activity. Nicholas Schou's book ''Orange Sunshine: The Brotherhood of Eternal Love and Its Quest to Spread Peace, Love, and Acid to the World'' describes one such group, ]. The group was a major American LSD trafficking group in the late 1960s and early 1970s.<ref>{{cite book| vauthors=Schou N |title=Orange Sunshine: The Brotherhood of Eternal Love and Its Quest to Spread Peace, Love, and Acid to the World |date=2010 |publisher=Thomas Dunne Books |url=https://archive.org/details/orangesunshinebr00scho_0 |url-access=registration |isbn=9780312551834}}</ref> | |||
| In the second half of the 20th century, dealers and chemists loosely associated with the ] like ], ], Karen Horning, Sarah Maltzer, "Dealer McDope," and ] played an essential role in distributing LSD.<ref name=Jarnow>{{cite book| vauthors=Jarnow J |title=Heads: A Biography of Psychedelic America |date=2016 |publisher=Da Capo Press |isbn=9780306822551}}</ref> | |||
| ====={{anchor|N-Bomb}} Mimics===== | |||
| ] | |||
| ] | |||
| Since 2005, law enforcement in the United States and elsewhere has seized several chemicals and combinations of chemicals in blotter paper which were sold as LSD mimics, including ],<ref name="microgram october 2005">{{Cite journal |journal=Microgram Bulletin |date=October 2005 |author=United States Drug Enforcement Administration |volume=38 |issue=10 |url=http://www.justice.gov/dea/pr/micrograms/2005/mg1005.pdf |title=LSD Blotter Acid Mimic Containing 4-Bromo-2,5-dimethoxy-amphetamine (DOB) Seized Near Burns, Oregon |access-date=August 20, 2009 |archive-url=https://web.archive.org/web/20121018052304/http://www.justice.gov/dea/pr/micrograms/2005/mg1005.pdf |archive-date=October 18, 2012}}</ref><ref name="microgram november 2006">{{Cite journal |journal=Microgram Bulletin |date=November 2006 |volume=39 |issue=11 |author=United States Drug Enforcement Administration |page=136 |title=Intelligence Alert – Blotter Acid Mimics (Containing 4-Bromo-2,5-Dimethoxy-Amphetamine (DOB)) in Concord, California |url=http://www.justice.gov/dea/pr/micrograms/2006/mg1106.pdf |access-date=August 20, 2009 |archive-url=https://web.archive.org/web/20121018052155/http://www.justice.gov/dea/pr/micrograms/2006/mg1106.pdf |archive-date=October 18, 2012}}</ref> a mixture of ] and ],<ref name="microgram march 2008">{{Cite journal |journal=Microgram Bulletin |date=March 2008 |volume=41 |issue=3 |author=United States Drug Enforcement Administration |url=http://www.justice.gov/dea/pr/micrograms/2008/mg0308.pdf |title=Unusual "Rice Krispie Treat"-Like Balls Containing Psilocybe Mushroom Parts in Warren County, Missouri |access-date=August 20, 2009 |archive-url=https://web.archive.org/web/20121017234315/http://www.justice.gov/dea/pr/micrograms/2008/mg0308.pdf |archive-date=October 17, 2012}}</ref> ],<ref name="ACMD Report">{{cite web| vauthors=Iversen L |title=Temporary Class Drug Order Report on 5-6APB and NBOMe compounds |url=https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/204808/J_TCDO_report_on_5-6APB_and_NBOMe_compounds.pdf |website=Advisory Council on the Misuse of Drugs |publisher=Gov.Uk |access-date=June 16, 2013 |date=May 29, 2013 |pages=14 |url-status=live |archive-url=https://web.archive.org/web/20130921234700/https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/204808/J_TCDO_report_on_5-6APB_and_NBOMe_compounds.pdf|archive-date=September 21, 2013}}</ref> and a mixture of ] and ].<ref name="microgram march 2009">{{Cite journal |journal=Microgram Bulletin |date=March 2009 |volume=42 |issue=3 |author=United States Drug Enforcement Administration |url=http://www.justice.gov/dea/pr/micrograms/2009/mg0309.pdf |title="Spice" – Plant Material(s) Laced With Synthetic Cannabinoids or Cannabinoid Mimicking Compounds |access-date=August 20, 2009 |archive-url=https://web.archive.org/web/20120118165818/http://www.justice.gov/dea/programs/forensicsci/microgram/mg0309/mg0309.html |archive-date=January 18, 2012}}</ref> Many mimics are toxic in comparatively small doses, or have extremely different safety profiles. Many street users of LSD are often under the impression that blotter paper which is actively hallucinogenic can only be LSD because that is the only chemical with low enough doses to fit on a small square of blotter paper. While it is true that LSD requires lower doses than most other hallucinogens, blotter paper is capable of absorbing a much larger amount of material. The DEA performed a ] analysis of blotter paper containing ] which showed that the paper contained a much greater concentration of the active chemical than typical LSD doses, although the exact quantity was not determined.<ref name="microgram november 2005">{{Cite journal |journal=Microgram Bulletin |date=November 2005 |volume=38 |issue=11 |author=United States Drug Enforcement Administration |url=http://www.justice.gov/dea/pr/micrograms/2005/mg1105.pdf |title=Bulk Marijuana in Hazardous Packaging in Chicago, Illinois |access-date=August 20, 2009 |archive-url=https://web.archive.org/web/20121018052300/http://www.justice.gov/dea/pr/micrograms/2005/mg1105.pdf |archive-date=October 18, 2012}}</ref> Blotter LSD mimics can have relatively small dose squares; a sample of blotter paper containing ] seized by ] police had dose markings approximately 6 mm apart.<ref name="microgram december 2007">{{Cite journal |journal=Microgram Bulletin |date=December 2007 |volume=40 |issue=12 |author=United States Drug Enforcement Administration |url=http://www.justice.gov/dea/pr/micrograms/2007/mg1207.pdf |title=SMALL HEROIN DISKS NEAR GREENSBORO, GEORGIA |access-date=August 20, 2009 |archive-url=https://web.archive.org/web/20121017234332/http://www.justice.gov/dea/pr/micrograms/2007/mg1207.pdf |archive-date=October 17, 2012}}</ref> Several deaths have been attributed to 25I-NBOMe.<ref name="Erowid25I-NBOMe">{{cite web |url=https://www.erowid.org/chemicals/2ci_nbome/2ci_nbome_death.shtml |title=25I-NBOMe (2C-I-NBOMe) Fatalities / Deaths |publisher=Erowid |access-date=February 28, 2016 |author=Erowid |url-status=live |archive-url=https://web.archive.org/web/20160305193143/https://www.erowid.org/chemicals/2ci_nbome/2ci_nbome_death.shtml |archive-date=March 5, 2016}}</ref><ref name="NY Daily news">{{cite news| vauthors=Hastings D |title=New drug N-bomb hits the street, terrifying parents, troubling cops |url=http://www.nydailynews.com/news/national/new-synthetic-hallucinogen-n-bomb-killing-users-cops-article-1.1336327 |access-date=May 7, 2013 |newspaper=New York Daily News |date=May 6, 2013 |url-status=live|archive-url=https://web.archive.org/web/20130510103039/http://www.nydailynews.com/news/national/new-synthetic-hallucinogen-n-bomb-killing-users-cops-article-1.1336327|archive-date=May 10, 2013}}</ref><ref name="Ireland injuries">{{cite news|vauthors=Feehan C |title=Powerful N-Bomb drug – responsible for spate of deaths internationally – responsible for hospitalisation of six in Cork |url=http://www.independent.ie/irish-news/powerful-nbomb-drug-responsible-for-spate-of-deaths-internationally-responsible-for-hospitalisation-of-six-in-cork-34384507.html|access-date=January 22, 2016|newspaper=Irish Independent|date=January 21, 2016 |archive-date=April 12, 2019|archive-url=https://web.archive.org/web/20190412203933/https://www.independent.ie/irish-news/powerful-nbomb-drug-responsible-for-spate-of-deaths-internationally-responsible-for-hospitalisation-of-six-in-cork-34384507.html|url-status=live}}</ref><ref name="ACMD Report2">{{cite web |vauthors=Iversen L |title=Temporary Class Drug Order Report on 5-6APB and NBOMe compounds |url=https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/204808/J_TCDO_report_on_5-6APB_and_NBOMe_compounds.pdf |website=Advisory Council on the Misuse of Drugs |publisher=Gov.Uk |access-date=June 16, 2013 |date=May 29, 2013 |url-status=live |archive-url=https://web.archive.org/web/20130921234700/https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/204808/J_TCDO_report_on_5-6APB_and_NBOMe_compounds.pdf |archive-date=September 21, 2013}}</ref> | |||
| ==Research== | |||
| In the United States, the earliest research began in the 1950s. ] and his colleagues published research on LSD's therapeutic potential to treat schizophrenia. In Canada, ] and Abram Hoffer completed LSD studies as early as 1952.<ref>{{Cite journal | vauthors = Dyck E |date=1965 |title=Flashback: Psychiatric Experimentation with LSD in Historical Perspective. |journal=Canadian Journal of Psychiatry |volume=50 |issue=7}}</ref> By the 1960s, controversies surrounding "hippie" counterculture began to deplete institutional support for continued studies. | |||
| Currently, several organizations—including ], ], ] and the ] Foundation—exist to fund, encourage and coordinate research into the medicinal and spiritual uses of LSD and related psychedelics.<ref>{{cite web |url=http://www.hofmann.org/ |title=The Albert Hofmann Foundation |access-date=September 27, 2007 |website=Hofmann Foundation |archive-url=https://web.archive.org/web/20190719174636/http://www.hofmann.org/ |archive-date=July 19, 2019}}</ref> New clinical LSD experiments in humans started in 2009 for the first time in 35 years.<ref name="new research">{{cite web|title=LSD-Assisted Psychotherapy |website=MAPS |url=http://www.maps.org/research/psilo-lsd |access-date=October 16, 2013|url-status=deviated |archive-url=https://web.archive.org/web/20180511060129/http://www.maps.org/research/psilo-lsd|archive-date=May 11, 2018}}</ref> As it is illegal in many areas of the world, potential medical uses are difficult to study.<ref name=Nutt2009/> | |||
| In 2001 the ] stated that LSD "produces no aphrodisiac effects, does not increase creativity, has no lasting positive effect in treating ] or criminals, does not produce a "]", and does not generate immediate personality change."<ref name="LSD: The Drug"/> More recently, experimental uses of LSD have included the treatment of alcoholism,<ref name=":0">{{cite journal |vauthors=Bogenschutz MP |date=March 2013 |title=Studying the effects of classic hallucinogens in the treatment of alcoholism: rationale, methodology, and current research with psilocybin |journal=Current Drug Abuse Reviews |volume=6 |issue=1 |pages=17–29 |pmid=23627783 |doi=10.2174/15733998113099990002}}</ref> pain and cluster headache relief,<ref name=Pas2008/><ref>{{Cite web | vauthors = Jarow O |date=2024-05-15 |title=Psychedelics could treat some of the worst chronic pain in the world |url=https://www.vox.com/future-perfect/2024/5/15/24156372/psychedelics-chronic-pain-cluster-headache-medicine-lsd-psilocybin |access-date=2024-05-25 |website=Vox |language=en-US |archive-date=May 25, 2024 |archive-url=https://web.archive.org/web/20240525084603/https://www.vox.com/future-perfect/2024/5/15/24156372/psychedelics-chronic-pain-cluster-headache-medicine-lsd-psilocybin |url-status=live }}</ref><ref>{{Cite web |title=RFA-AG-25-004: Safety and Early Efficacy Studies of Psychedelic-Assisted Therapy for Chronic Pain in Older Adults (UG3/UH3 Clinical Trial Required) |url=https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-25-004.html |access-date=2024-05-25 |website=grants.nih.gov |archive-date=May 25, 2024 |archive-url=https://web.archive.org/web/20240525084606/https://grants.nih.gov/grants/guide/rfa-files/RFA-AG-25-004.html |url-status=live }}</ref> and prospective studies on depression.<ref>{{Cite journal |title=LSD Therapy for Persons Suffering From Major Depression - Full Text View |url=https://clinicaltrials.gov/ct2/show/NCT03866252 |access-date=2021-03-09 |website=ClinicalTrials.gov |date=February 8, 2021 |language=en |archive-date=June 11, 2021 |archive-url=https://web.archive.org/web/20210611214216/https://clinicaltrials.gov/ct2/show/NCT03866252 |url-status=live}}</ref> | |||
| A 2020 meta-review indicated possible positive effects of LSD in reducing psychiatric symptoms, mainly in cases of alcoholism.<ref>{{cite journal |title=Therapeutic Use of LSD in Psychiatry: A Systematic Review of Randomized-Controlled Clinical Trials |vauthors=Fuentes JJ, Fonseca F, Elices M, Farré M, Torrens M |pmid=32038315 |pmc=6985449 |journal=Frontiers in Psychiatry |doi=10.3389/fpsyt.2019.00943 |volume=10 |date=January 2020 |page=943 |doi-access=free}}</ref> There is evidence that psychedelics induce molecular and cellular adaptations related to neuroplasticity and that these could potentially underlie therapeutic benefits.<ref name="pmid36123427">{{cite journal |vauthors=Calder AE, Hasler G |title=Towards an understanding of psychedelic-induced neuroplasticity |journal=Neuropsychopharmacology |volume=48 |issue=1 |pages=104–112 |date=January 2023 |pmid=36123427 |pmc=9700802 |doi=10.1038/s41386-022-01389-z |doi-access=free}}</ref><ref name="pmid35060714">{{cite journal |vauthors=Olson DE |title=Biochemical Mechanisms Underlying Psychedelic-Induced Neuroplasticity |journal=Biochemistry |volume=61 |issue=3 |pages=127–136 |date=February 2022 |pmid=35060714 |pmc=9004607 |doi=10.1021/acs.biochem.1c00812}}</ref> | |||
| ===Psychedelic therapy=== | |||
| {{see also|Psychedelic therapy}} | |||
| In the 1950s and 1960s, LSD was used in psychiatry to enhance psychotherapy, known as ]. Some psychiatrists, such as ], who pioneered its use at ] in England, believed LSD was especially useful at helping patients to "unblock" repressed subconscious material through other ] methods,<ref>Cohen, S. (1959). "The therapeutic potential of LSD-25". ''A Pharmacologic Approach to the Study of the Mind'', p. 251–258.</ref> and also for treating alcoholism.<ref>{{cite journal |title=Use of d-Lysergic Acid Diethylamide in the Treatment of Alcoholism |journal=Q. J. Stud. Alcohol |volume=20 |pages=577–590 |vauthors=Chwelos N, Blewett DB, Smith CM, Hoffer A |date=1959 |issue=3 |doi=10.15288/qjsa.1959.20.577 |pmid=13810249 |url=http://www.erowid.org/references/texts/show/1852docid1733 |access-date=June 20, 2012 |archive-url=https://web.archive.org/web/20210224135151/https://www.erowid.org/references/texts/show/1852docid1733 |archive-date=February 24, 2021 |url-status=live}} Via {{cite web |title=Abstract |url=http://www.hofmann.org/papers/blewett_1.html |access-date=February 22, 2012 |url-status=live |archive-url=https://web.archive.org/web/20120203022019/http://www.hofmann.org/papers/blewett_1.html |archive-date=February 3, 2012 |website=Hofmann.org}}</ref><ref>{{Cite journal |vauthors=Frood A |date=2012-03-09 |title=LSD helps to treat alcoholism |journal=Nature News |url=http://www.nature.com/news/lsd-helps-to-treat-alcoholism-1.10200 |doi=10.1038/nature.2012.10200 |s2cid=137367650 |access-date=December 25, 2020 |archive-date=March 8, 2021 |archive-url=https://web.archive.org/web/20210308135250/https://www.nature.com/news/lsd-helps-to-treat-alcoholism-1.10200 |url-status=live}}</ref> One study concluded, "The root of the therapeutic value of the LSD experience is its potential for producing ] and self-surrender,"<ref name="Use of d-lysergic acid diethylamide"/> presumably by forcing the user to face issues and problems in that individual's psyche. | |||
| Two recent reviews concluded that conclusions drawn from most of these early trials are unreliable due to serious ] flaws. These include the absence of adequate ], lack of follow-up, and vague criteria for ] outcome. In many cases, studies failed to convincingly demonstrate whether the drug or the therapeutic interaction was responsible for any beneficial effects.<ref>{{cite journal |vauthors=Vollenweider FX, Kometer M |title=The neurobiology of psychedelic drugs: implications for the treatment of mood disorders |journal=Nature Reviews. Neuroscience |volume=11 |issue=9 |pages=642–51 |date=September 2010 |pmid=20717121 |doi=10.1038/nrn2884 |s2cid=16588263}}</ref><ref>{{cite journal |vauthors=Baumeister D, Barnes G, Giaroli G, Tracy D |title=Classical hallucinogens as antidepressants? A review of pharmacodynamics and putative clinical roles |journal=Therapeutic Advances in Psychopharmacology |volume=4 |issue=4 |pages=156–69 |date=August 2014 |pmid= 25083275 |pmc=4104707 |doi=10.1177/2045125314527985}}</ref> | |||
| In recent years, organizations like the ] (MAPS) have renewed clinical research of LSD.<ref name="new research"/> | |||
| It has been proposed that LSD be studied for use in the therapeutic setting, particularly in anxiety.<ref name=":5"/><ref name=":6"/><ref name="clinicalLSD">{{cite journal |vauthors=Liechti ME |title=Modern Clinical Research on LSD |journal=Neuropsychopharmacology |volume=42 |issue=11 |pages=2114–2127 |date=October 2017 |pmid=28447622 |pmc=5603820 |doi=10.1038/npp.2017.86}}</ref><ref>{{Cite news |title=Psychedelics are transforming the way we understand depression and its treatment |date=April 20, 2021 |newspaper=The Guardian |vauthors=Carhart-Harris R |url=https://www.theguardian.com/commentisfree/2021/apr/20/psychedelics-depression-treatment-psychiatry-psilocybin |access-date=16 May 2021 |archive-url=https://web.archive.org/web/20210611212734/https://www.theguardian.com/commentisfree/2021/apr/20/psychedelics-depression-treatment-psychiatry-psilocybin|archive-date=June 11, 2021|url-status=live}}</ref> In 2024, the FDA designated a form of LSD as a breakthrough therapy to treat ] which is being developed by ].<ref>{{Cite web | vauthors = Terry K |date=2024-03-26 |title=FDA Opens the Door to Clinical Use of LSD |url=https://www.webmd.com/mental-health/news/20240326/fda-opens-the-door-clinical-use-lsd |access-date=2024-05-25 |website=WebMD |language=en |archive-date=May 25, 2024 |archive-url=https://web.archive.org/web/20240525075810/https://www.webmd.com/mental-health/news/20240326/fda-opens-the-door-clinical-use-lsd |url-status=live }}</ref> | |||
| ===Other uses=== | |||
| In the 1950s and 1960s, some psychiatrists (e.g., ]) explored the potential effect of LSD on creativity. Experimental studies attempted to measure the effect of LSD on creative activity and aesthetic appreciation.<ref name="PMID6054248"/><ref name="PMID18562421">{{cite journal |vauthors=Sessa B |title=Is it time to revisit the role of psychedelic drugs in enhancing human creativity? |journal=Journal of Psychopharmacology |volume=22 |issue=8 |pages=821–827 |date=November 2008 |pmid=18562421 |s2cid=1908638 |doi=10.1177/0269881108091597}}</ref><ref name="PMID2723891">{{cite journal |vauthors=Janiger O, Dobkin de Rios M |title=LSD and creativity |journal=Journal of Psychoactive Drugs |volume=21 |issue=1 |pages=129–134 |year=1989 |pmid=2723891 |doi=10.1080/02791072.1989.10472150 |url= http://www.erowid.org/culture/characters/janiger_oscar/janiger_oscar.shtml |archive-url=https://web.archive.org/web/20091003005533/http://www.erowid.org/culture/characters/janiger_oscar/janiger_oscar.shtml |archive-date=October 3, 2009 |url-status=live}}</ref><ref name="Stafford-Golightly">{{cite book |title=LSD, the problem-solving psychedelic |vauthors=Stafford PG, Golightly BH |year=1967 |url=http://www.psychedelic-library.org/staf3.htm |url-status=live |archive-url=https://web.archive.org/web/20120417105503/http://www.psychedelic-library.org//staf3.htm |archive-date =April 17, 2012}}</ref> In 1966 Dr. James Fadiman conducted a study with the central question "How can psychedelics be used to facilitate problem solving?" This study attempted to solve 44 different problems and had 40 satisfactory solutions when the FDA banned all research into psychedelics. LSD was a key component of this study.<ref>{{cite web |title=Scientific Problem Solving with Psychedelics – James Fadiman |website=] |date=May 29, 2013 |url=https://www.youtube.com/watch?v=KtL5fafpRKc |access-date=2023-05-02 |language=en |archive-date=September 8, 2019 |archive-url=https://web.archive.org/web/20190908090741/https://www.youtube.com/watch?v=KtL5fafpRKc |url-status=live }}</ref><ref>{{cite book |vauthors=Fadiman J |title=The psychedelic explorer's guide: safe, therapeutic, and sacred journeys |date=2018 |publisher=Tantor Media |isbn=978-1-9773-7476-9 |oclc=1031461623}}</ref> | |||
| Since 2008 there has been ongoing research into using LSD to alleviate anxiety for ] cancer patients coping with their impending deaths.<ref name=":5" /><ref name="new research"/><ref>{{Cite news |url=http://bazonline.ch/wissen/medizin-und-psychologie/Psychiater-Gasser-bricht-sein-Schweigen/story/25732295|title=Psychiater Gasser bricht sein Schweigen|date=July 28, 2009 |newspaper=Basler Zeitung|access-date=June 19, 2011|archive-url=https://web.archive.org/web/20111006122541/http://bazonline.ch/wissen/medizin-und-psychologie/Psychiater-Gasser-bricht-sein-Schweigen/story/25732295|archive-date=October 6, 2011}}</ref> | |||
| A 2012 meta-analysis found evidence that a single dose of LSD in conjunction with various alcoholism treatment programs was associated with a decrease in alcohol abuse, lasting for several months, but no effect was seen at one year. Adverse events included seizure, moderate ] and agitation, nausea, ], and acting in a bizarre fashion.<ref name="Lysergic acid diethylamide LSD fo"/> | |||
| LSD has been used as a treatment for ]s with positive results in some small studies.<ref name=Pas2008/> | |||
| LSD is a potent ], a compound capable of promoting rapid and sustained ] that may have wide-ranging therapeutic benefit.<ref name=Ly2018>{{cite journal |title=Psychedelics promote structural and functional neural plasticity |journal=Cell Reports |vauthors=Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, Burbach KF, Soltanzadeh Zarandi S, Sood A, Paddy MR, Duim WC, Dennis MY, McAllister AK, Ori-McKenney KM, Gray JA, Olson DE |year=2018 |volume=23 |issue=11 |pages=3170–3182 |doi=10.1016/j.celrep.2018.05.022 |pmid=29898390 |pmc=6082376 }}</ref> LSD has been shown to increase markers of neuroplasticity in human brain ]s and improve memory performance in human subjects.<ref>{{Cite news |vauthors=Dolan EW |date=2022-08-11 |title=Neuroscience research suggests LSD might enhance learning and memory by promoting brain plasticity |url=https://www.psypost.org/2022/08/neuroscience-research-suggests-lsd-might-enhance-learning-and-memory-by-promoting-brain-plasticity-63701 |access-date=2022-09-12 |newspaper=Psypost - Psychology News |language=en-US |archive-date=August 26, 2022 |archive-url=https://web.archive.org/web/20220826005449/https://www.psypost.org/2022/08/neuroscience-research-suggests-lsd-might-enhance-learning-and-memory-by-promoting-brain-plasticity-63701 |url-status=live }}</ref> | |||
| LSD may have analgesic properties related to pain in terminally ill patients and ] and may be useful for treating inflammatory diseases including rheumatoid arthritis.<ref name=lsdpain>{{cite journal |vauthors=Whelan A, Johnson MI |title=Lysergic acid diethylamide and psilocybin for the management of patients with persistent pain: a potential role? |journal=Pain Management |volume=8 |issue=3 |pages=217–229 |date=May 2018 |pmid=29722608 |doi=10.2217/pmt-2017-0068 |s2cid=19160293 |url=http://eprints.leedsbeckett.ac.uk/4843/1/LysergicAcidDiethylamide%28LSD%29andPsilocybinAM-JOHNSON.pdf |access-date=August 22, 2020 |archive-date=October 8, 2020 |archive-url=https://web.archive.org/web/20201008201515/http://eprints.leedsbeckett.ac.uk/4843/1/LysergicAcidDiethylamide%28LSD%29andPsilocybinAM-JOHNSON.pdf |url-status=live}}</ref> | |||
| ==Notable individuals== | |||
| Some notable individuals have commented publicly on their experiences with LSD.<ref>{{cite web |title=Famous LSD users |url=http://www.thegooddrugsguide.com/articles/famous_users/lsd.htm |publisher=The Good Drugs Guide |access-date=2008-10-20 |url-status=live |archive-url=https://web.archive.org/web/20081007023344/http://www.thegooddrugsguide.com/articles/famous_users/lsd.htm |archive-date=October 7, 2008}}</ref><ref>{{cite web |url=http://popwiki.net |title=People on psychedelics |access-date=2012-11-01 |url-status=live |archive-url=https://web.archive.org/web/20130421145334/http://popwiki.net/ |archive-date=April 21, 2013}}</ref> Some of these comments date from the era when it was legally available in the US and Europe for non-medical uses, and others pertain to ] treatment in the 1950s and 1960s. Still others describe experiences with illegal LSD, obtained for philosophic, artistic, therapeutic, spiritual, or recreational purposes. | |||
| * ], the poet, said, "I myself have taken mescaline once and L.S.D. once. Aside from a slight schizophrenic dissociation of the I from the Not-I, including my body, nothing happened at all."<ref>{{cite journal | vauthors = Mason D | title = Review: Awe for Auden | journal = The Hudson Review | volume = 68 | issue = 3 | date = Autumn 2015 | pages = 492–500 | publisher = The Hudson Review, Inc. }}</ref> He also said, "LSD was a complete frost. … What it does seem to destroy is the power of communication. I have listened to tapes done by highly articulate people under LSD, for example, and they talk absolute drivel. They may have seen something interesting, but they certainly lose either the power or the wish to communicate."<ref>{{cite web | url = http://www.swarthmore.edu/library/auden/QandA_pt6.html | vauthors = Auden WH | title = W. H. Auden at Swathmore; An hour of questions and answers with Auden | date = 15 November 1971 | work = Exhibition notes from the W.H. Auden Collection | publisher = the Swarthmore College Library | access-date = February 23, 2021 | archive-date = June 11, 2021 | archive-url = https://web.archive.org/web/20210611212901/http://www.swarthmore.edu/library/auden/QandA_pt6.html | url-status = live }}</ref> He also said, "Nothing much happened but I did get the distinct impression that some birds were trying to communicate with me."<ref>{{cite news | url = https://www.irishtimes.com/news/a-master-of-memorable-speech-1.1195982 | vauthors = MacMonagle N | title = A Master of Memorable speech | newspaper = The Irish Times | date = 17 February 2007 }}</ref> | |||
| * ], an American peace activist, says he has had several hundred experiences with psychedelics.<ref>{{Cite web| vauthors=Meyer A |date=2022-01-24 |title=Daniel Ellsberg Talks Psychedelics, Consciousness and World Peace |url=https://www.lucid.news/daniel-ellsberg-talks-psychedelics-consciousness-and-world-peace/|access-date=2022-01-29|website=Lucid News|language=en-US|archive-date=January 29, 2022|archive-url=https://web.archive.org/web/20220129143452/https://www.lucid.news/daniel-ellsberg-talks-psychedelics-consciousness-and-world-peace/|url-status=live}}</ref> | |||
| * ], a notable physicist at ], tried LSD during his professorship at Caltech. Feynman largely sidestepped the issue when dictating his anecdotes; he mentions it in passing in the "O Americano, Outra Vez" section.<ref>{{cite book | vauthors = Feynman RP |title=Surely You're Joking, Mr. Feynman!: Adventures of a Curious Character | veditors = Leighton R |publisher=] |year=1985 |isbn=978-0-393-01921-6 |oclc=10925248|title-link=Surely You're Joking, Mr. Feynman! }}</ref><ref>{{cite book | vauthors = Gleick J |author-link=James Gleick |title=Genius: The Life and Science of Richard Feynman |publisher=] |year=1992|isbn=978-0-679-40836-9 |oclc=243743850}}</ref> | |||
| * ] stated in a July 3, 1989 interview for '']'', in response to the question "Have your feelings about LSD changed over the years?," "They haven't changed much. My feelings about LSD are mixed. It's something that I both fear and that I love at the same time. I never take any psychedelic, have a psychedelic experience, without having that feeling of, "I don't know what's going to happen." In that sense, it's still fundamentally an enigma and a mystery."<ref>{{cite web|url=http://www.relix.com/features/2010/04/20/q-a-with-jerry-garcia-portrait-of-an-artist-as-a-tripper?3 |title=Q&A with Jerry Garcia: Portrait of an Artist as a Tripper |publisher=Relix Magazine |date=April 20, 2010 |access-date=2013-06-29 | vauthors = Alderson J |archive-url=https://web.archive.org/web/20100521033451/http://www.relix.com/features/2010/04/20/q-a-with-jerry-garcia-portrait-of-an-artist-as-a-tripper?3 |archive-date=May 21, 2010}}</ref> | |||
| * ] implied in an interview with '']'' that he tried LSD during his youth.<ref>{{cite magazine |title= The Bill Gates Interview |magazine= Playboy |date= July 1994 |url= http://www.playboy.com/playground/view/50-years-of-the-playboy-interview-bill-gates |archive-url= https://web.archive.org/web/20140707190401/http://www.playboy.com/playground/view/50-years-of-the-playboy-interview-bill-gates |archive-date= July 7, 2014}}</ref> | |||
| * ], author of '']'', became a user of psychedelics after moving to ]. He was at the forefront of the counterculture's use of psychedelic drugs, which led to his 1954 work '']''. Dying from cancer, he asked his wife on 22 November 1963 to inject him with 100 μg of LSD. He died later that day.<ref name=OpenCulture>{{cite news |vauthors=Colman D |title=Aldous Huxley's LSD Death Trip |newspaper=Open Culture |date=October 2011 |url=http://www.openculture.com/2011/10/aldous_huxleys_lsd_death_trip.html |access-date=1 November 2011 |url-status=live |archive-url=https://web.archive.org/web/20111112140024/http://www.openculture.com/2011/10/aldous_huxleys_lsd_death_trip.html |archive-date=November 12, 2011}}</ref> | |||
| * ], co-founder and former CEO of ], said, "Taking LSD was a profound experience, one of the most important things in my life."<ref>{{Cite news | vauthors = Bosker B |title=The Steve Jobs Reading List: The Books And Artists That Made The Man |newspaper=Huffington Post |url=http://www.huffingtonpost.com/2011/10/21/the-steve-jobs-reading-list-the-books_n_1024021.html |access-date=23 October 2011 |date=21 October 2011 |url-status=live |archive-url=https://web.archive.org/web/20111022123850/http://www.huffingtonpost.com/2011/10/21/the-steve-jobs-reading-list-the-books_n_1024021.html |archive-date=October 22, 2011}}</ref> | |||
| * ], German writer and philosopher, throughout his life had experimented with ] such as ], ], and ]; and later in life he used ] and LSD. These experiments were recorded comprehensively in '']'' (1970, ''Approaches''). The novel '']'' (1952, ''Visit to Godenholm'') is clearly influenced by his early experiments with mescaline and LSD. He met with LSD inventor ] and they took LSD together several times. Hofmann's memoir ''LSD, My Problem Child'' describes some of these meetings.<ref>{{cite web|title=LSD, My Problem Child · Radiance from Ernst Junger|website=www.psychedelic-library.org |url=http://www.psychedelic-library.org/child7.htm |access-date=April 17, 2021|archive-date=May 12, 2021|archive-url=https://web.archive.org/web/20210512173700/http://www.psychedelic-library.org/child7.htm|url-status=live}}</ref> | |||
| * In a 2004 interview, ] said that ]' songs "]" and "]" were inspired by LSD trips.<ref name=Sheff/>{{rp|182}} Nonetheless, ] consistently stated over the course of many years that the fact that the initials of "Lucy in the Sky with Diamonds" spelled out L-S-D was a coincidence (he stated that the title came from a picture drawn by his son ]) and that the band members did not notice until after the song had been released, and Paul McCartney corroborated that story.<ref>{{cite web |date=1998-02-15 |title=Is 'Lucy in the Sky with Diamonds' Code for LSD? |url=https://www.snopes.com/fact-check/lucy-in-the-sky-with-diamonds/ |access-date=2012-06-20 |website=Snopes.com |publisher= |archive-date=December 20, 2021 |archive-url=https://web.archive.org/web/20211220152957/https://www.snopes.com/fact-check/lucy-in-the-sky-with-diamonds/ |url-status=live }}</ref> ], ], and ] also used the drug, although McCartney cautioned that "it's easy to overestimate the influence of drugs on the Beatles' music."<ref name="weeklystandard">{{cite magazine |title=The Truth Behind "LSD" |vauthors=Matus V |date=June 2004 |magazine=The Weekly Standard |url=https://www.washingtonexaminer.com/weekly-standard/the-truth-behind-lsd |access-date=November 3, 2019 |url-status=live |archive-date=March 8, 2021 |archive-url=https://web.archive.org/web/20210308185326/https://www.washingtonexaminer.com/weekly-standard/the-truth-behind-lsd}}</ref> | |||
| *] had an LSD experience with Simeon Wade in ] and later wrote "it was the greatest experience of his life, and that it profoundly changed his life and his work."<ref>{{Cite web|url=http://www.openculture.com/2017/09/when-michel-foucault-tripped-on-acid-in-death-valley-and-called-it-the-greatest-experience-of-my-life-1975.html|title=When Michel Foucault Tripped on Acid in Death Valley and Called It "The Greatest Experience of My Life"|date=September 1975|website=Open Culture|language=en-US|access-date=2019-04-27|archive-date=March 15, 2021|archive-url=https://web.archive.org/web/20210315225234/https://openculture.com/2017/09/when-michel-foucault-tripped-on-acid-in-death-valley-and-called-it-the-greatest-experience-of-my-life-1975.html|url-status=live}}</ref><ref>{{Cite news |url=https://lareviewofbooks.org/article/blowing-the-philosophers-fuses-michel-foucaults-lsd-trip-in-the-valley-of-death/|title=Blowing The Philosopher's Fuses: Michel Foucault's LSD Trip in The Valley of Death|vauthors=Penner J|website=Los Angeles Review of Books|date=June 17, 2019 |language=en-US|access-date=11 April 2021|archive-date=April 11, 2021|archive-url=https://web.archive.org/web/20210411165642/https://lareviewofbooks.org/article/blowing-the-philosophers-fuses-michel-foucaults-lsd-trip-in-the-valley-of-death/#_ftnref4|url-status=live}} Wade: "We fell silent to listen to Stockhausen's '']''. Zabriskie Point was filled with the sound of a kindergarten playground overlaid with electric tonalities. ''Kontakte'' followed. ]s bounced off the stars, which glowed like incandescent pinballs. Foucault turned to Michael and said this is the first time he really understood what Stockhausen had achieved".</ref> According to Wade, as soon as he came back to Paris, Foucault scrapped the second History of Sexuality's manuscript, and totally rethought the whole project.<ref>{{Cite book|title=Foucault in California: | vauthors=Wade S |publisher=Heyday Books |year=2019 |isbn=9781597144636}} In a letter to Wade, dated 16 September 1978, Foucault authorised the book's publication and added: "How could I not love you?"</ref> | |||
| * ] is reported to credit LSD with helping him develop ] technology, for which he received the ] in 1993.<ref>{{Cite magazine| url=https://www.wired.com/science/discoveries/news/2006/01/70015 |title=LSD: The Geek's Wonder Drug? |access-date=2008-03-11 |vauthors=Harrison A |date=2006-01-16 |magazine=Wired |quote=Like Herbert, many scientists and engineers also report heightened states of creativity while using LSD. During a press conference on Friday, Hofmann revealed that he was told by Nobel-prize-winning chemist Kary Mullis that LSD had helped him develop the polymerase chain reaction that helps amplify specific DNA sequences. |url-status=live |archive-url=https://web.archive.org/web/20080505100508/http://www.wired.com/science/discoveries/news/2006/01/70015 |archive-date=May 5, 2008}}</ref> | |||
| * ], an Italian ] and writer, has credited his use of LSD with sparking his interest in theoretical physics.<ref>{{Cite web|vauthors=Higgins C|date=2018-04-14|title='There is no such thing as past or future': physicist Carlo Rovelli on changing how we think about time |website=The Guardian |url=http://www.theguardian.com/books/2018/apr/14/carlo-rovelli-exploding-commonsense-notions-order-of-time-interview |access-date=2022-02-06|archive-date=January 11, 2022|archive-url=https://web.archive.org/web/20220111094136/https://www.theguardian.com/books/2018/apr/14/carlo-rovelli-exploding-commonsense-notions-order-of-time-interview|url-status=live}}</ref> | |||
| * ], a ] famous for writing best-selling case histories about his patients' disorders and unusual experiences, talks about his own experiences with LSD and other perception altering chemicals, in his book, '']''.<ref>{{cite book |vauthors=Sacks O |date=2012 |title=Hallucinations |publisher=] |page=106 |url=https://www.oliversacks.com/books-by-oliver-sacks/hallucinations/ |isbn=978-0-307-94743-7 |quote=On the West Coast in the early 1960s LSD and morning glory seeds were readily available, so I sampled those, too. |access-date=June 30, 2018 |url-status=live |archive-date=April 21, 2021 |archive-url=https://web.archive.org/web/20210421064209/https://www.oliversacks.com/books-by-oliver-sacks/hallucinations/}}</ref> | |||
| * ] and ], creators of the TV series '']'', claimed to have shown up at the ], at which they were nominated for Best Original Song, under the influence of LSD.<ref>{{cite web | vauthors = Bose SD |title=When Trey Parker and Matt Stone went to the Oscars on LSD Swapnil Dhruv Bose |date=December 27, 2021 |website=FarOutMagazine.co.uk |url=https://faroutmagazine.co.uk/when-trey-parker-and-matt-stone-went-to-the-oscars-on-lsd/ |access-date=January 20, 2022 |url-status=live |archive-date=January 20, 2022 |archive-url=https://web.archive.org/web/20220120001258/https://faroutmagazine.co.uk/when-trey-parker-and-matt-stone-went-to-the-oscars-on-lsd/}}</ref> | |||
| == See also == | == See also == | ||
| {{portal|1960s}} | |||
| * ] | |||
| {{div col |colwidth=15em}} | |||
| * ], deaths mistakenly attributed to LSD overdoses | |||
| * ] | * ] | ||
| * ] | * ] | ||
| * '']'' (ergot) | |||
| * ] | |||
| * ] | |||
| * ], ] mind-control research program that began in the 1950s. | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| {{div col end}} | |||
| * ], US Supreme Court case | |||
| * Related ] compounds: ]s, ], ], ], ] | |||
| == |
== Notes == | ||
| {{ |
{{notelist}} | ||
| == |
== References == | ||
| {{reflist|refs= | |||
| * Aldous FAB, Barrass BC, Brewster K, et al. ''Journal of Medicinal Chemistry'', Vol. 17, 1100–1111 (1974) | |||
| <ref name=Dol2015>{{cite journal |vauthors=Dolder PC, Schmid Y, Haschke M, Rentsch KM, Liechti ME |title=Pharmacokinetics and Concentration-Effect Relationship of Oral LSD in Humans |journal=The International Journal of Neuropsychopharmacology |volume=19 |issue=1 |pages=pyv072 |date=June 2015 |pmid=26108222 |pmc=4772267 |doi=10.1093/ijnp/pyv072}}</ref> | |||
| *Grof, Stanislave. ''LSD Psychotherapy''. (April 10, 2001) | |||
| <ref name=Muc2016>{{cite journal |vauthors=Mucke HA |title=From Psychiatry to Flower Power and Back Again: The Amazing Story of Lysergic Acid Diethylamide |journal=Assay and Drug Development Technologies |volume=14 |issue=5 |pages=276–281 |date=July 2016 |pmid=27392130 |doi=10.1089/adt.2016.747}}</ref> | |||
| *Lee, Martin A. and Bruce Shlain. ''Acid Dreams: The Complete Social History of LSD: The CIA, the Sixties, and Beyond'' | |||
| }} | |||
| == Further reading == | |||
| * {{cite book | title = LSD: My Problem Child | author = ] | url = https://books.google.com/books?id=e5wQAQAAIAAJ | year = 1980| publisher = McGraw-Hill | isbn = 978-0-07-029325-0 }} | |||
| == External links == | == External links == | ||
| {{wikiquote|LSD}} | |||
| {{Wikinews|Drug website surveys LSD users and culture}} | |||
| {{commons}} | |||
| * Dr. Albert Hofmann - The father of LSD | |||
| * {{Webarchive|url=https://web.archive.org/web/20081015023231/http://www.erowid.org/chemicals/lsd/lsd.shtml |date=October 15, 2008 }} at ] | |||
| * , April 16, 2001 | |||
| * {{Webarchive|url=https://web.archive.org/web/20151118232537/https://www.erowid.org/library/books_online/tihkal/tihkal26.shtml |date=November 18, 2015 }} at ] by ] | |||
| * "", lecture by Jack Cowan (via the ]) | |||
| * {{Webarchive|url=https://web.archive.org/web/20220831090729/https://psychonautwiki.org/LSD |date=August 31, 2022 }} at PsychonautWiki | |||
| *"" by the National Film Board of Canada | |||
| ===Documentaries=== | |||
| * | |||
| * {{Webarchive|url=https://web.archive.org/web/20210616120006/http://www.nfb.ca/film/hofmanns_potion/ |date=June 16, 2021 }} a documentary on the origins of LSD, 2002 | |||
| * | |||
| * National Geographic Channel, 2009 | |||
| * , ''Science & Consciousness Review'' | |||
| * '' {{Webarchive|url=https://web.archive.org/web/20220616180919/https://www.netflix.com/de-en/title/80229847 |date=June 16, 2022 }}'' Netflix docuseries, 2022 | |||
| * | |||
| * | |||
| * | |||
| * | |||
| {{ChemicalSources}} | |||
| {{Hallucinogenic lysergamides}} | |||
| {{Chemical agents}} | |||
| ] | |||
| {{Drug use}} | |||
| {{Hallucinogens}} | |||
| {{Navboxes | |||
| | title = ] | |||
| | titlestyle = background:#ccccff | |||
| | list1 = | |||
| {{Adrenergic receptor modulators}} | |||
| {{Dopamine receptor modulators}} | |||
| {{Serotonin receptor modulators}} | |||
| }} | |||
| {{Ergolines}} | |||
| {{Authority control}} | |||
| {{DEFAULTSORT:Lysergic Acid Diethylamide}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 00:31, 8 January 2025
Hallucinogenic drug"Lsd" redirects here. Not to be confused with £sd. For other uses, see LSD (disambiguation).
Pharmaceutical compound
 Skeletal formula of LSD Skeletal formula of LSD | |
| Clinical data | |
|---|---|
| Pronunciation | /daɪ eθəl ˈæmaɪd/, /æmɪd/, or /eɪmaɪd/ |
| Trade names | Delysid |
| Other names | LSD, LSD-25, LAD, acid, lucy, among others |
| AHFS/Drugs.com | Reference |
| Pregnancy category |
|
| Dependence liability | Low |
| Addiction liability | None |
| Routes of administration | By mouth, sublingual |
| Drug class | Serotonergic psychedelic (hallucinogen) |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 71% |
| Protein binding | Unknown |
| Metabolism | Liver (CYP450) |
| Metabolites | 2-Oxo-3-hydroxy-LSD |
| Onset of action | 30–40 minutes |
| Elimination half-life | 3.6 hours |
| Duration of action | 8–20 hours |
| Excretion | Kidneys |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.031 |
| Chemical and physical data | |
| Formula | C20H25N3O |
| Molar mass | 323.440 g·mol |
| 3D model (JSmol) | |
| Melting point | 80 to 85 °C (176 to 185 °F) |
| Solubility in water | 67.02 mg/mL (20 °C) |
SMILES
| |
InChI
| |
| (verify) | |
Lysergic acid diethylamide, commonly known as LSD (from German Lysergsäure-diethylamid), is a potent psychedelic drug that intensifies thoughts, emotions, and sensory perception. Often referred to as acid or lucy, LSD can cause mystical, spiritual, or religious experiences. At higher doses, it primarily induces visual and auditory hallucinations. LSD is not considered addictive, because it does not produce compulsive drug-seeking behavior. Using LSD can lead to adverse psychological reactions, such as anxiety, paranoia, and delusions. Additionally, it may trigger "flashbacks," also known as hallucinogen persisting perception disorder (HPPD), where individuals experience persistent visual distortions after use.
The effects of LSD begin within 30 minutes of ingestion and can last up to 20 hours, with most trips averaging 8–12 hours. It is synthesized from lysergic acid and commonly administered via tabs of blotter paper. LSD is mainly used recreationally or for spiritual purposes. As a serotonin receptor agonist, LSD's precise effects are not fully understood, but it is known to alter the brain’s default mode network, leading to its powerful psychedelic effects.
The drug was first synthesized by Swiss chemist Albert Hofmann in 1938 and became widely studied in the 1950s and 1960s. It was used experimentally in psychiatry for treating alcoholism and schizophrenia. However, its association with the counterculture movement of the 1960s led to its classification as a Schedule I drug in the U.S. in 1968. It was also listed as a Schedule I controlled substance by the United Nations in 1971 and remains without approved medical uses.
Despite its legal restrictions, LSD remains influential in scientific and cultural contexts. Its therapeutic potential has been explored, particularly in treating mental health disorders. As of 2017, about 10% of people in the U.S. had used LSD at some point, with 0.7% having used it in the past year. Usage rates have risen, with a 56.4% increase in adult use in the U.S. from 2015 to 2018.
Uses
Recreational
LSD is commonly used as a recreational drug.
Spiritual
LSD can catalyze intense spiritual experiences and is thus considered an entheogen. Some users have reported out of body experiences. In 1966, Timothy Leary established the League for Spiritual Discovery with LSD as its sacrament. Stanislav Grof has written that religious and mystical experiences observed during LSD sessions appear to be phenomenologically indistinguishable from similar descriptions in the sacred scriptures of the great religions of the world and the texts of ancient civilizations.
Medical
See also: Lysergic acid diethylamide § ResearchLSD currently has no approved uses in medicine. A meta analysis concluded that a single dose was shown to be effective at reducing alcohol consumption in people suffering from alcoholism. LSD has also been studied in depression, anxiety, and drug dependence, with positive preliminary results.
Effects
LSD is exceptionally potent, with as little as 20 μg capable of producing a noticeable effect.
Physical


LSD can induce physical effects such as pupil dilation, decreased appetite, increased sweating, and wakefulness. The physical reactions to LSD vary greatly and some may be a result of its psychological effects. Commonly observed symptoms include increased body temperature, blood sugar, and heart rate, as well as goose bumps, jaw clenching, dry mouth, and hyperreflexia. In cases of adverse reactions, users may experience numbness, weakness, nausea, and tremors.
Psychological
The primary immediate psychological effects of LSD are visual pseudo-hallucinations and altered thought, often referred to as "trips". These sensory alterations are considered pseudohallucinations because the subject does not perceive the patterns seen as being located in three-dimensional space outside the body. LSD is not considered addictive. These effects typically begin within 20–30 minutes of oral ingestion, peak three to four hours after ingestion, and can last up to 20 hours, particularly with higher doses. An "afterglow" effect, characterized by an improved mood or perceived mental state, may persist for days or weeks following ingestion. Positive experiences, or "good trips", are described as intensely pleasurable and can include feelings of joy, euphoria, an increased appreciation for life, decreased anxiety, a sense of spiritual enlightenment, and a feeling of interconnectedness with the universe.
Negative experiences, commonly known as "bad trips", can induce feelings of fear, agitation, anxiety, panic, and paranoia. While the occurrence of a bad trip is unpredictable, factors such as mood, surroundings, sleep, hydration, and social setting, collectively referred to as "set and setting", can influence the risk and are considered important in minimizing the likelihood of a negative experience.
Sensory
LSD induces an animated sensory experience affecting senses, emotions, memories, time, and awareness, lasting from 6 to 20 hours, with the duration dependent on dosage and individual tolerance. Effects typically commence within 30 to 90 minutes post-ingestion, ranging from subtle perceptual changes to profound cognitive shifts. Alterations in auditory and visual perception are common.
Users may experience enhanced visual phenomena, such as vibrant colors, objects appearing to morph, ripple or move, and geometric patterns on various surfaces. Changes in the perception of food's texture and taste are also noted, sometimes leading to aversion towards certain foods.
There are reports of inanimate objects appearing animated, with static objects seeming to move in additional spatial dimensions. The auditory effects of LSD may include echo-like distortions of sounds, and an intensified experience of music. Basic visual effects often resemble phosphenes and can be influenced by concentration, thoughts, emotions, or music. Higher doses can lead to more intense sensory perception alterations, including synesthesia, perception of additional dimensions, and temporary dissociation.
Adverse effects
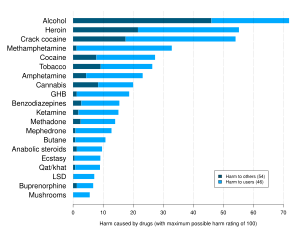

LSD, a classical psychedelic, is deemed physiologically safe at standard dosages (50–200 μg) and its primary risks lie in psychological effects rather than physiological harm. A 2010 study by David Nutt ranked LSD as significantly less harmful than alcohol, placing it near the bottom of a list assessing the harm of 20 drugs.
Psychological effects
Mental disorders
LSD can induce panic attacks or extreme anxiety, colloquially termed a "bad trip". Despite lower rates of depression and substance abuse found in psychedelic drug users compared to controls, LSD presents heightened risks for individuals with severe mental illnesses like schizophrenia. These hallucinogens can catalyze psychiatric disorders in predisposed individuals, although they do not tend to induce illness in emotionally healthy people.
Suggestibility
While research from the 1960s indicated increased suggestibility under the influence of LSD among both mentally ill and healthy individuals, recent documents suggest that the CIA and Department of Defense have discontinued research into LSD as a means of mind control.
Flashbacks
Flashbacks are psychological episodes where individuals re-experience some of LSD's subjective effects after the drug has worn off, persisting for days or months post-hallucinogen use. These experiences are associated with hallucinogen persisting perception disorder (HPPD), where flashbacks occur intermittently or chronically, causing distress or functional impairment.
The etiology of flashbacks is varied. Some cases are attributed to somatic symptom disorder, where individuals fixate on normal somatic experiences previously unnoticed prior to drug consumption. Other instances are linked to associative reactions to contextual cues, similar to responses observed in individuals with past trauma or emotional experiences. The risk factors for flashbacks remain unclear, but pre-existing psychopathologies may be significant contributors.
Estimating the prevalence of HPPD is challenging. It is considered rare, with occurrences ranging from 1 in 20 users experiencing the transient and less severe type 1 HPPD, to 1 in 50,000 for the more concerning type 2 HPPD. Contrary to internet rumors, LSD is not stored long-term in the spinal cord or other body parts. Pharmacological evidence indicates LSD has a half-life of 175 minutes and is metabolized into water-soluble compounds like 2-oxo-3-hydroxy-LSD, eliminated through urine without evidence of long-term storage. Clinical evidence also suggests that chronic use of SSRIs can potentiate LSD-induced flashbacks, even months after stopping LSD use.
Drug interactions
Several psychedelics, including LSD, are metabolized by CYP2D6. Concurrent use of SSRIs, potent inhibitors of CYP2D6, with LSD may heighten the risk of serotonin syndrome. Chronic usage of SSRIs, TCAs, and MAOIs is believed to diminish the subjective effects of psychedelics, likely due to SSRI-induced 5-HT2A receptor downregulation and MAOI-induced 5-HT2A receptor desensitization. Interactions between psychedelics and antipsychotics or anticonvulsants are not well-documented; however, co-use with mood stabilizers like lithium may induce seizures and dissociative effects, particularly in individuals with bipolar disorder. Lithium notably intensifies LSD reactions, potentially leading to acute comatose states when combined.
Lethal dose
The lethal oral dose of LSD in humans is estimated at 100 mg, based on LD50 and lethal blood concentrations observed in rodent studies.
Tolerance
LSD shows significant tachyphylaxis, with tolerance developing 24 hours after administration. The progression of tolerance at intervals shorter than 24 hours remains largely unknown. Tolerance typically resets to baseline after 3–4 days of abstinence. Significant cross-tolerance occurs between LSD, mescaline and psilocybin. A slight cross-tolerance to DMT is observed in humans highly tolerant to LSD. Tolerance to LSD also builds up with consistent use, and is believed to result from serotonin 5-HT2A receptor downregulation. Researchers believe that tolerance returns to baseline after two weeks of not using psychedelics.
Addiction and dependence liability
LSD is widely considered to be non-addictive, despite its potential for abuse. Attempts to train laboratory animals to self-administer LSD have been largely unsuccessful. Although tolerance to LSD builds up rapidly, a withdrawal syndrome does not appear, suggesting that a potential syndrome does not necessarily relate to the possibility of acquiring rapid tolerance to a substance. A report examining substance use disorder for DSM-IV noted that almost no hallucinogens produced dependence, unlike psychoactive drugs of other classes such as stimulants and depressants.
Cancer and pregnancy
The mutagenic potential of LSD is unclear. Overall, the evidence seems to point to limited or no effect at commonly used doses. Studies showed no evidence of teratogenic or mutagenic effects.
Overdose
There have been no documented fatal human overdoses from LSD, although there has been no "comprehensive review since the 1950s" and "almost no legal clinical research since the 1970s". Eight individuals who had accidentally consumed an exceedingly high amount of LSD, mistaking it for cocaine, and had gastric levels of 1000–7000 μg LSD tartrate per 100 mL and blood plasma levels up to 26 μg/ml, had suffered from comatose states, vomiting, respiratory problems, hyperthermia, and light gastrointestinal bleeding; however, all of them survived without residual effects upon hospital intervention.
Individuals experiencing a bad trip after LSD intoxication may present with severe anxiety and tachycardia, often accompanied by phases of psychotic agitation and varying degrees of delusions. Cases of death on a bad trip have been reported due to prone maximal restraint (commonly known as a hogtie) and positional asphyxia when the individuals were restrained by law enforcement personnel.
Massive doses are largely managed by symptomatic treatments, and agitation can be addressed with benzodiazepines. Reassurance in a calm, safe environment is beneficial. Antipsychotics such as haloperidol are not recommended as they may have adverse psychotomimetic effects. Gastrointestinal decontamination with activated charcoal is of little use due to the rapid absorption of LSD, unless done within 30–60 minutes of ingesting exceedingly huge amounts. Administration of anticoagulants, vasodilators, and sympatholytics may be useful for treating ergotism.
Designer drug overdose
Many novel psychoactive substances of 25-NB (NBOMe) series, such as 25I-NBOMe and 25B-NBOMe, are regularly sold as LSD in blotter papers. NBOMe compounds are often associated with life-threatening toxicity and death. Fatalities involved in NBOMe intoxication suggest that a significant number of individuals ingested the substance which they believed was LSD, and researchers report that "users familiar with LSD may have a false sense of security when ingesting NBOMe inadvertently". Researchers state that the alleged physiological toxicity of LSD is likely due to psychoactive substances other than LSD.
NBOMe compounds are reported to have a bitter taste, are not active orally, and are usually taken sublingually. When NBOMes are administered sublingually, numbness of the tongue and mouth followed by a metallic chemical taste was observed, and researchers describe this physical side effect as one of the main discriminants between NBOMe compounds and LSD. Despite its high potency, recreational doses of LSD have only produced low incidents of acute toxicity, but NBOMe compounds have extremely different safety profiles. Testing with Ehrlich's reagent gives a positive result for LSD and a negative result for NBOMe compounds.
Pharmacology
Pharmacodynamics

| Target | Affinity (Ki, nM) |
|---|---|
| 5-HT1A | 0.64–7.3 |
| 5-HT1B | 3.9 |
| 5-HT1D | 3.9–14 |
| 5-HT1E | 93 |
| 5-HT1F | ND |
| 5-HT2A | 0.48–21 (Ki) 0.24–225 (EC50Tooltip half-maximal effective concentration) 23–84% (EmaxTooltip maximal efficacy) |
| 5-HT2B | 0.98–30 (Ki) 8.9–12,000 (EC50) 13–71% (Emax) |
| 5-HT2C | 1.1–48 (Ki) 0.85 (EC50) 26–79% (Emax) |
| 5-HT3 | >10,000 |
| 5-HT4 | 1,000 (rat) |
| 5-HT5A | 9.0 |
| 5-HT5B | 3.2 (rat) |
| 5-HT6 | 2.3–6.9 |
| 5-HT7 | 6.3–6.6 |
| α1A | 670–1,128 |
| α1B | 8,677 |
| α1D | ND |
| α2A | 12–46 |
| α2B | ND |
| α2C | ND |
| β1 | 140–1,601 |
| β2 | 740–3,461 |
| β3 | ND |
| D1 | 177–340 |
| D2 | 110–126 |
| D3 | 27 |
| D4 | 56–158 |
| D5 | 344 |
| H1 | 1,100–1,540 |
| H2–H4 | ND |
| M1–M5 | ND |
| I1 | ND |
| σ1 | ND |
| σ2 | ND |
| TAAR1 | 450 (Ki) (rat) 10,000 (Ki) (mouse) 1,400 (EC50) (rat) 9,700 (EC50) (mouse) >20,000 (EC50) (human) |
| SERTTooltip Serotonin transporter | >30,000 (Ki) >100,000 (IC50Tooltip half-maximal inhibitory concentration) |
| NETTooltip Norepinephrine transporter | 5,600–>30,000 (Ki) >100,000 (IC50) |
| DATTooltip Dopamine transporter | >30,000 (Ki) >100,000 (IC50) |
| Notes: The smaller the value, the more avidly the drug binds to the site. All proteins are human unless otherwise noted. Refs: | |
Most serotonergic psychedelics are not significantly dopaminergic, and LSD is therefore atypical in this regard. The agonism of the D2 receptor by LSD may contribute to its psychoactive effects in humans.
LSD binds to most serotonin receptor subtypes except for the 5-HT3 and 5-HT4 receptors. However, most of these receptors are affected at too low affinity to be sufficiently activated by the brain concentration of approximately 10–20 nM. In humans, recreational doses of LSD can affect 5-HT1A (Ki = 1.1 nM), 5-HT2A (Ki = 2.9 nM), 5-HT2B (Ki = 4.9 nM), 5-HT2C (Ki = 23 nM), 5-HT5A (Ki = 9 nM ), and 5-HT6 receptors (Ki = 2.3 nM). Although not present in humans, 5-HT5B receptors found in rodents also have a high affinity for LSD. The psychedelic effects of LSD are attributed to cross-activation of 5-HT2A receptor heteromers. Many but not all 5-HT2A agonists are psychedelics and 5-HT2A antagonists block the psychedelic activity of LSD. LSD exhibits functional selectivity at the 5-HT2A and 5-HT2C receptors in that it activates the signal transduction enzyme phospholipase A2 instead of activating the enzyme phospholipase C as the endogenous ligand serotonin does.
Exactly how LSD produces its effects is unknown, but it is thought that it works by increasing glutamate release in the cerebral cortex and therefore excitation in this area, specifically in layer V. LSD, like many other drugs of recreational use, has been shown to activate DARPP-32-related pathways. The drug enhances dopamine D2 receptor protomer recognition and signaling of D2–5-HT2A receptor complexes, which may contribute to its psychotropic effects. LSD has been shown to have low affinity for H1 receptors, displaying antihistamine effects.
LSD is a biased agonist that induces a conformation in serotonin receptors that preferentially recruits β-arrestin over activating G proteins. LSD also has an exceptionally long residence time when bound to serotonin receptors lasting hours, consistent with the long-lasting effects of LSD despite its relatively rapid clearance. A crystal structure of 5-HT2B bound to LSD reveals an extracellular loop that forms a lid over the diethylamide end of the binding cavity which explains the slow rate of LSD unbinding from serotonin receptors. The related lysergamide lysergic acid amide (LSA) that lacks the diethylamide moiety is far less hallucinogenic in comparison.
LSD, like other psychedelics, has been found to increase the expression of genes related to synaptic plasticity. This is in part due to binding to brain-derived neurotrophic factor (BDNF) receptor TrkB.
Mechanisms of action
 Resting state fMRI BOLD-contrast imaging shows increased primary visual cortex (V1) cerebral blood flow (CBF) and increased V1 resting state functional connectivity (RSFC), which correlated more strongly with the visual hallucinatory aspect of the LSD experience. Increased V1 RSFC also correlated with visual analogue scale (VAS) ratings of simple hallucinations and the magnitude of CBF observed in the visual cortex correlated positively with ratings of complex imagery on the LSD-induced altered state of consciousness (ASC).
Resting state fMRI BOLD-contrast imaging shows increased primary visual cortex (V1) cerebral blood flow (CBF) and increased V1 resting state functional connectivity (RSFC), which correlated more strongly with the visual hallucinatory aspect of the LSD experience. Increased V1 RSFC also correlated with visual analogue scale (VAS) ratings of simple hallucinations and the magnitude of CBF observed in the visual cortex correlated positively with ratings of complex imagery on the LSD-induced altered state of consciousness (ASC). Resting state fMRI BOLD-contrast imaging shows decreased bilateral parahippocampal (PH) resting state functional connectivity (RSFC), which correlated with the ego-dissolution aspect of the LSD experience. A significant relationship was also found between decreased posterior cingulate cortex (PCC) alpha power and default mode network (DMN) disintegration with ego-dissolution.
Resting state fMRI BOLD-contrast imaging shows decreased bilateral parahippocampal (PH) resting state functional connectivity (RSFC), which correlated with the ego-dissolution aspect of the LSD experience. A significant relationship was also found between decreased posterior cingulate cortex (PCC) alpha power and default mode network (DMN) disintegration with ego-dissolution.
Neuroimaging studies using resting state fMRI recently suggested that LSD changes the cortical functional architecture. These modifications spatially overlap with the distribution of serotoninergic receptors. In particular, increased connectivity and activity were observed in regions with high expression of 5-HT2A receptor, while a decrease in activity and connectivity was observed in cortical areas that are dense with 5-HT1A receptor. Experimental data suggest that subcortical structures, particularly the thalamus, play a synergistic role with the cerebral cortex in mediating the psychedelic experience. LSD, through its binding to cortical 5-HT2A receptor, may enhance excitatory neurotransmission along frontostriatal projections and, consequently, reduce thalamic filtering of sensory stimuli towards the cortex. This phenomenon appears to selectively involve ventral, intralaminar, and pulvinar nuclei.
Pharmacokinetics
The acute effects of LSD normally last between 6 and 10 hours depending on dosage, tolerance, and age. Aghajanian and Bing (1964) found LSD had an elimination half-life of only 175 minutes (about 3 hours). However, using more accurate techniques, Papac and Foltz (1990) reported that 1 μg/kg oral LSD given to a single male volunteer had an apparent plasma half-life of 5.1 hours, with a peak plasma concentration of 5 ng/mL at 3 hours post-dose.
The pharmacokinetics of LSD were not properly determined until 2015, which is not surprising for a drug with the kind of low-μg potency that LSD possesses. In a sample of 16 healthy subjects, a single mid-range 200 μg oral dose of LSD was found to produce mean maximal concentrations of 4.5 ng/mL at a median of 1.5 hours (range 0.5–4 hours) post-administration. Concentrations of LSD decreased following first-order kinetics with a half-life of 3.6±0.9 hours and a terminal half-life of 8.9±5.9 hours.
The effects of the dose of LSD given lasted for up to 12 hours and were closely correlated with the concentrations of LSD present in circulation over time, with no acute tolerance observed. Only 1% of the drug was eliminated in urine unchanged, whereas 13% was eliminated as the major metabolite 2-oxo-3-hydroxy-LSD (O-H-LSD) within 24 hours. O-H-LSD is formed by cytochrome P450 enzymes, although the specific enzymes involved are unknown, and it does not appear to be known whether O-H-LSD is pharmacologically active or not. The oral bioavailability of LSD was crudely estimated as approximately 71% using previous data on intravenous administration of LSD. The sample was equally divided between male and female subjects and there were no significant sex differences observed in the pharmacokinetics of LSD.
Chemistry

LSD is a chiral compound with two stereocenters at the carbon atoms C-5 and C-8, so that theoretically four different optical isomers of LSD could exist. LSD, also called (+)-d-LSD, has the absolute configuration (5R,8R). 5S stereoisomers of lysergamides do not exist in nature and are not formed during the synthesis from d-lysergic acid. Retrosynthetically, the C-5 stereocenter could be analysed as having the same configuration of the alpha carbon of the naturally occurring amino acid L-tryptophan, the precursor to all biosynthetic ergoline compounds.
However, LSD and iso-LSD, the two C-8 isomers, rapidly interconvert in the presence of bases, as the alpha proton is acidic and can be deprotonated and reprotonated. Non-psychoactive iso-LSD which has formed during the synthesis can be separated by chromatography and can be isomerized to LSD.
Pure salts of LSD are triboluminescent, emitting small flashes of white light when shaken in the dark. LSD is strongly fluorescent and will glow bluish-white under UV light.
Synthesis
LSD is an ergoline derivative. It is commonly synthesized by reacting diethylamine with an activated form of lysergic acid. Activating reagents include phosphoryl chloride and peptide coupling reagents. Lysergic acid is made by alkaline hydrolysis of lysergamides like ergotamine, a substance usually derived from the ergot fungus on agar plate. Lysergic acid can also be produced synthetically, although these processes are not used in clandestine manufacture due to their low yields and high complexity.
Albert Hofmann synthesized LSD in the following manner: (1) hydrazinolysis of ergotamine into D- and L-isolysergic acid hydrazide, (2) seperation of the enantiomers with di-(p-touyl)-D-tartaric acid to get D-isolysergic acid hydrazide, (3) enantiomerization into D-lysergic acid hydrazide, (4) substitution with HNO2 to D-lysergic acid azide and (5) finally substitution with diethylamine to form D-lysergic acid diethylamide.
Research
The precursor for LSD, lysergic acid, has been produced by GMO baker's yeast.
Dosage

A single dose of LSD is typically between 40 and 500 micrograms—an amount roughly equal to one-tenth the mass of a grain of sand. Threshold effects can be felt with as little as 25 micrograms of LSD. The practice of using sub-threshold doses is called microdosing. Dosages of LSD are measured in micrograms (μg), or millionths of a gram.
In the mid-1960s, the most important black market LSD manufacturer (Owsley Stanley) distributed LSD at a standard concentration of 270 μg, while street samples of the 1970s contained 30 to 300 μg. By the 1980s, the amount had reduced to between 100 and 125 μg, dropping more in the 1990s to the 20–80 μg range, and even more in the 2000s (decade).
Reactivity and degradation
"LSD," writes the chemist Alexander Shulgin, "is an unusually fragile molecule ... As a salt, in water, cold, and free from air and light exposure, it is stable indefinitely."
LSD has two labile protons at the tertiary stereogenic C5 and C8 positions, rendering these centers prone to epimerisation. The C8 proton is more labile due to the electron-withdrawing carboxamide attachment, but the removal of the chiral proton at the C5 position (which was once also an alpha proton of the parent molecule tryptophan) is assisted by the inductively withdrawing nitrogen and pi electron delocalisation with the indole ring.
LSD also has enamine-type reactivity because of the electron-donating effects of the indole ring. Because of this, chlorine destroys LSD molecules on contact; even though chlorinated tap water contains only a slight amount of chlorine, the small quantity of compound typical to an LSD solution will likely be eliminated when dissolved in tap water. The double bond between the 8-position and the aromatic ring, being conjugated with the indole ring, is susceptible to nucleophilic attacks by water or alcohol, especially in the presence of UV or other kinds of light. LSD often converts to "lumi-LSD," which is inactive in human beings.
A controlled study was undertaken to determine the stability of LSD in pooled urine samples. The concentrations of LSD in urine samples were followed over time at various temperatures, in different types of storage containers, at various exposures to different wavelengths of light, and at varying pH values. These studies demonstrated no significant loss in LSD concentration at 25 °C for up to four weeks. After four weeks of incubation, a 30% loss in LSD concentration at 37 °C and up to a 40% at 45 °C were observed. Urine fortified with LSD and stored in amber glass or nontransparent polyethylene containers showed no change in concentration under any light conditions. The stability of LSD in transparent containers under light was dependent on the distance between the light source and the samples, the wavelength of light, exposure time, and the intensity of light. After prolonged exposure to heat in alkaline pH conditions, 10 to 15% of the parent LSD epimerized to iso-LSD. Under acidic conditions, less than 5% of the LSD was converted to iso-LSD. It was also demonstrated that trace amounts of metal ions in the buffer or urine could catalyze the decomposition of LSD and that this process can be avoided by the addition of EDTA.
Detection

LSD can be detected in concentrations larger than approximately 10% in a sample using Ehrlich's reagent and Hofmann's reagent. However, detecting LSD in human tissues is more challenging due to its active dosage being significantly lower (in micrograms) compared to most other drugs (in milligrams).
LSD may be quantified in urine for drug testing programs, in plasma or serum to confirm poisoning in hospitalized victims, or in whole blood for forensic investigations. The parent drug and its major metabolite are unstable in biofluids when exposed to light, heat, or alkaline conditions, necessitating protection from light, low-temperature storage, and quick analysis to minimize losses. Maximum plasma concentrations are typically observed 1.4 to 1.5 hours after oral administration of 100 μg and 200 μg, respectively, with a plasma half-life of approximately 2.6 hours (ranging from 2.2 to 3.4 hours among test subjects).
Due to its potency in microgram quantities, LSD is often not included in standard pre-employment urine or hair analyses. However, advanced liquid chromatography–mass spectrometry methods can detect LSD in biological samples even after a single use.
History
—Albert Hofmann, on his first experience with LSD Main article: History of LSD... affected by a remarkable restlessness, combined with a slight dizziness. At home I lay down and sank into a not unpleasant intoxicated-like condition, characterized by an extremely stimulated imagination. In a dreamlike state, with eyes closed (I found the daylight to be unpleasantly glaring), I perceived an uninterrupted stream of fantastic pictures, extraordinary shapes with intense, kaleidoscopic play of colors. After some two hours this condition faded away.
Swiss chemist Albert Hofmann first synthesized LSD in 1938 from lysergic acid, a chemical derived from the hydrolysis of ergotamine, an alkaloid found in ergot, a fungus that infects grain. LSD was the 25th of various lysergamides Hofmann synthesized from lysergic acid while trying to develop a new analeptic, hence the alternate name LSD-25. Hofmann discovered its effects in humans in 1943, after unintentionally ingesting an unknown amount, possibly absorbing it through his skin. LSD was subject to exceptional interest within the field of psychiatry in the 1950s and early 1960s, with Sandoz distributing LSD to researchers under the trademark name Delysid in an attempt to find a marketable use for it. During this period, LSD was controversially administered to hospitalised schizophrenic autistic children, with varying degrees of therapeutic success.
LSD-assisted psychotherapy was used in the 1950s and early 1960s by psychiatrists such as Humphry Osmond, who pioneered the application of LSD to the treatment of alcoholism, with promising results. Osmond coined the term "psychedelic" (lit. mind manifesting) as a term for LSD and related hallucinogens, superseding the previously held "psychotomimetic" model in which LSD was believed to mimic schizophrenia. In contrast to schizophrenia, LSD can induce transcendent experiences, or mental states that transcend the experience of everyday consciousness, with lasting psychological benefit. During this time, the Central Intelligence Agency (CIA) began using LSD in the research project Project MKUltra, which used psychoactive substances to aid interrogation. The CIA administered LSD to unwitting test subjects to observe how they would react, the most well-known example of this being Operation Midnight Climax. LSD was one of several psychoactive substances evaluated by the U.S. Army Chemical Corps as possible non-lethal incapacitants in the Edgewood Arsenal human experiments.
In the 1960s, LSD and other psychedelics were adopted by and became synonymous with, the counterculture movement due to their perceived ability to expand consciousness. This resulted in LSD being viewed as a cultural threat to American values and the Vietnam war effort, and it was designated as a Schedule I (illegal for medical as well as recreational use) substance in 1968. It was listed as a Schedule I controlled substance by the United Nations in 1971 and currently has no approved medical uses. As of 2017, about 10% of people in the United States have used LSD at some point in their lives, while 0.7% have used it in the last year. It was most popular in the 1960s to 1980s. The use of LSD among US adults increased by 56.4% from 2015 to 2018.
LSD was first synthesized on November 16, 1938 by Swiss chemist Albert Hofmann at the Sandoz Laboratories in Basel, Switzerland as part of a large research program searching for medically useful ergot alkaloid derivatives. The abbreviation "LSD" is from the German "Lysergsäurediethylamid".

LSD's psychedelic properties were discovered 5 years later when Hofmann himself accidentally ingested an unknown quantity of the chemical. The first intentional ingestion of LSD occurred on April 19, 1943, when Hofmann ingested 250 μg of LSD. He said this would be a threshold dose based on the dosages of other ergot alkaloids. Hofmann found the effects to be much stronger than he anticipated. Sandoz Laboratories introduced LSD as a psychiatric drug in 1947 and marketed LSD as a psychiatric panacea, hailing it "as a cure for everything from schizophrenia to criminal behavior, 'sexual perversions', and alcoholism." Sandoz would send the drug for free to researchers investigating its effects.
Beginning in the 1950s, the US Central Intelligence Agency (CIA) began a research program code-named Project MKUltra. The CIA introduced LSD to the United States, purchasing the entire world's supply for $240,000 and propagating the LSD through CIA front organizations to American hospitals, clinics, prisons, and research centers. Experiments included administering LSD to CIA employees, military personnel, doctors, other government agents, prostitutes, mentally ill patients, and members of the general public to study their reactions, usually without the subjects' knowledge. The project was revealed in the US congressional Rockefeller Commission report in 1975.
In 1963, the Sandoz patents on LSD expired and the Czech company Spofa began to produce the substance. Sandoz stopped the production and distribution in 1965.
Several figures, including Aldous Huxley, Timothy Leary, and Al Hubbard, had begun to advocate the consumption of LSD. LSD became central to the counterculture of the 1960s. In the early 1960s the use of LSD and other hallucinogens was advocated by new proponents of consciousness expansion such as Leary, Huxley, Alan Watts and Arthur Koestler, and according to L. R. Veysey they profoundly influenced the thinking of the new generation of youth.
On October 24, 1968, possession of LSD was made illegal in the United States. The last FDA approved study of LSD in patients ended in 1980, while a study in healthy volunteers was made in the late 1980s. Legally approved and regulated psychiatric use of LSD continued in Switzerland until 1993.
In November 2020, Oregon became the first US state to decriminalize possession of small amounts of LSD after voters approved Ballot Measure 110.
Society and culture
Counterculture
By the mid-1960s, the youth countercultures in California, particularly in San Francisco, had widely adopted the use of hallucinogenic drugs, including LSD. The first major underground LSD factory was established by Owsley Stanley. Around this time, the Merry Pranksters, associated with novelist Ken Kesey, organized the Acid Tests, events in San Francisco involving LSD consumption, accompanied by light shows and improvised music. Their activities, including cross-country trips in a psychedelically decorated bus and interactions with major figures of the beat movement, were later documented in Tom Wolfe's The Electric Kool-Aid Acid Test (1968).
In San Francisco's Haight-Ashbury neighborhood, the Psychedelic Shop was opened in January 1966 by brothers Ron and Jay Thelin to promote the safe use of LSD. This shop played a significant role in popularizing LSD in the area and establishing Haight-Ashbury as the epicenter of the hippie counterculture. The Thelins also organized the Love Pageant Rally in Golden Gate Park in October 1966, protesting against California's ban on LSD.
A similar movement developed in London, led by British academic Michael Hollingshead, who first tried LSD in America in 1961. After experiencing LSD and interacting with notable figures such as Aldous Huxley, Timothy Leary, and Richard Alpert, Hollingshead played a key role in the famous LSD research at Millbrook before moving to New York City for his experiments. In 1965, he returned to the UK and founded the World Psychedelic Center in Chelsea, London.
Music and Art

The influence of LSD in the realms of music and art became pronounced in the 1960s, especially through the Acid Tests and related events involving bands like the Grateful Dead, Jefferson Airplane, and Big Brother and the Holding Company. San Francisco-based artists such as Rick Griffin, Victor Moscoso, and Wes Wilson contributed to this movement through their psychedelic poster and album art. The Grateful Dead, in particular, became central to the culture of "Deadheads," with their music heavily influenced by LSD.
In the United Kingdom, Michael Hollingshead, reputed for introducing LSD to various artists and musicians like Storm Thorgerson, Donovan, Keith Richards, and members of the Beatles, played a significant role in the drug's proliferation in the British art and music scene. Despite LSD's illegal status from 1966, it was widely used by groups including the Beatles, the Rolling Stones, and the Moody Blues. Their experiences influenced works such as the Beatles' Sgt. Pepper's Lonely Hearts Club Band and Cream's Disraeli Gears, featuring psychedelic-themed music and artwork.
Psychedelic music of the 1960s often sought to replicate the LSD experience, incorporating exotic instrumentation, electric guitars with effects pedals, and elaborate studio techniques. Artists and bands utilized instruments like sitars and tablas, and employed studio effects such as backward tapes, panning, and phasing. Songs such as John Prine's "Illegal Smile" and the Beatles' "Lucy in the Sky with Diamonds" have been associated with LSD, although the latter's authors denied such claims.
Contemporary artists influenced by LSD include Keith Haring in the visual arts, various electronic dance music creators, and the jam band Phish. The 2018 Leo Butler play All You Need is LSD is inspired by the author's interest in the history of LSD.
Legal status
The United Nations Convention on Psychotropic Substances of 1971 mandates that signing parties, including the United States, Australia, New Zealand, and most of Europe, prohibit LSD. Enforcement of these laws varies by country. The convention allows medical and scientific research with LSD.
Australia
In Australia, LSD is classified as a Schedule 9 prohibited substance under the Poisons Standard (February 2017), indicating it may be abused or misused and its manufacture, possession, sale, or use should be prohibited except for approved research purposes. In Western Australia, the Misuse of Drugs Act 1981 provides guidelines for possession and trafficking of substances like LSD.
Canada
In Canada, LSD is listed under Schedule III of the Controlled Drugs and Substances Act. Unauthorized possession and trafficking of the substance can lead to significant legal penalties.
United Kingdom
In the United Kingdom, LSD is a Class A drug under the Misuse of Drugs Act 1971, making unauthorized possession and trafficking punishable by severe penalties. The Runciman Report and Transform Drug Policy Foundation have made recommendations and proposals regarding the legal regulation of LSD and other psychedelics.
United States
In the United States, LSD is classified as a Schedule I controlled substance under the Controlled Substances Act of 1970, making its manufacture, possession, and distribution illegal without a DEA license. The law considers LSD to have a high potential for abuse, no legitimate medical use, and to be unsafe even under medical supervision. The US Supreme Court case Neal v. United States (1995) clarified the sentencing guidelines related to LSD possession.
Oregon decriminalized personal possession of small amounts of drugs, including LSD, in February 2021, and California has seen legislative efforts to decriminalize psychedelics.
Mexico
Mexico decriminalized the possession of small amounts of drugs, including LSD, for personal use in 2009. The law specifies possession limits and establishes that possession is not a crime within designated quantities.
Czech Republic
In the Czech Republic, possession of "amount larger than small" of LSD is criminalized, while possession of smaller amounts is a misdemeanor. The definition of "amount larger than small" is determined by judicial practice and specific regulations.
Economics
Production

An active dose of LSD is very minute, allowing a large number of doses to be synthesized from a comparatively small amount of raw material. Twenty-five kilograms of precursor ergotamine tartrate can produce 5–6 kg of pure crystalline LSD; this corresponds to around 50–60 million doses at 100 μg. Because the masses involved are so small, concealing and transporting illicit LSD is much easier than smuggling cocaine, cannabis, or other illegal drugs.
Manufacturing LSD requires laboratory equipment and experience in the field of organic chemistry. It takes two to three days to produce 30 to 100 grams of pure compound. It is believed that LSD is not usually produced in large quantities, but rather in a series of small batches. This technique minimizes the loss of precursor chemicals in case a step does not work as expected.
Forms

LSD is produced in crystalline form and is then mixed with excipients or redissolved for production in ingestible forms. Liquid solution is either distributed in small vials or, more commonly, sprayed onto or soaked into a distribution medium. Historically, LSD solutions were first sold on sugar cubes, but practical considerations forced a change to tablet form. Appearing in 1968 as an orange tablet measuring about 6 mm across, "Orange Sunshine" acid was the first largely available form of LSD after its possession was made illegal. Tim Scully, a prominent chemist, made some of these tablets, but said that most "Sunshine" in the USA came by way of Ronald Stark, who imported approximately thirty-five million doses from Europe.
Over some time, tablet dimensions, weight, shape and concentration of LSD evolved from large (4.5–8.1 mm diameter), heavyweight (≥150 mg), round, high concentration (90–350 μg/tab) dosage units to small (2.0–3.5 mm diameter) lightweight (as low as 4.7 mg/tab), variously shaped, lower concentration (12–85 μg/tab, average range 30–40 μg/tab) dosage units. LSD tablet shapes have included cylinders, cones, stars, spacecraft, and heart shapes. The smallest tablets became known as "Microdots."
After tablets came "computer acid" or "blotter paper LSD," typically made by dipping a preprinted sheet of blotting paper into an LSD/water/alcohol solution. More than 200 types of LSD tablets have been encountered since 1969 and more than 350 blotter paper designs have been observed since 1975. About the same time as blotter paper LSD came "Windowpane" (AKA "Clearlight"), which contained LSD inside a thin gelatin square a quarter of an inch (6 mm) across. LSD has been sold under a wide variety of often short-lived and regionally restricted street names including Acid, Trips, Uncle Sid, Blotter, Lucy, Alice and doses, as well as names that reflect the designs on the sheets of blotter paper. Authorities have encountered the drug in other forms—including powder or crystal, and capsule.
Modern distribution
LSD manufacturers and traffickers in the United States can be categorized into two groups: A few large-scale producers, and an equally limited number of small, clandestine chemists, consisting of independent producers who, operating on a comparatively limited scale, can be found throughout the country.
As a group, independent producers are of less concern to the Drug Enforcement Administration than the large-scale groups because their product reaches only local markets.
Many LSD dealers and chemists describe a religious or humanitarian purpose that motivates their illicit activity. Nicholas Schou's book Orange Sunshine: The Brotherhood of Eternal Love and Its Quest to Spread Peace, Love, and Acid to the World describes one such group, the Brotherhood of Eternal Love. The group was a major American LSD trafficking group in the late 1960s and early 1970s.
In the second half of the 20th century, dealers and chemists loosely associated with the Grateful Dead like Owsley Stanley, Nicholas Sand, Karen Horning, Sarah Maltzer, "Dealer McDope," and Leonard Pickard played an essential role in distributing LSD.
Mimics


Since 2005, law enforcement in the United States and elsewhere has seized several chemicals and combinations of chemicals in blotter paper which were sold as LSD mimics, including DOB, a mixture of DOC and DOI, 25I-NBOMe, and a mixture of DOC and DOB. Many mimics are toxic in comparatively small doses, or have extremely different safety profiles. Many street users of LSD are often under the impression that blotter paper which is actively hallucinogenic can only be LSD because that is the only chemical with low enough doses to fit on a small square of blotter paper. While it is true that LSD requires lower doses than most other hallucinogens, blotter paper is capable of absorbing a much larger amount of material. The DEA performed a chromatographic analysis of blotter paper containing 2C-C which showed that the paper contained a much greater concentration of the active chemical than typical LSD doses, although the exact quantity was not determined. Blotter LSD mimics can have relatively small dose squares; a sample of blotter paper containing DOC seized by Concord, California police had dose markings approximately 6 mm apart. Several deaths have been attributed to 25I-NBOMe.
Research
In the United States, the earliest research began in the 1950s. Albert Kurland and his colleagues published research on LSD's therapeutic potential to treat schizophrenia. In Canada, Humphry Osmond and Abram Hoffer completed LSD studies as early as 1952. By the 1960s, controversies surrounding "hippie" counterculture began to deplete institutional support for continued studies.
Currently, several organizations—including the Beckley Foundation, MAPS, Heffter Research Institute and the Albert Hofmann Foundation—exist to fund, encourage and coordinate research into the medicinal and spiritual uses of LSD and related psychedelics. New clinical LSD experiments in humans started in 2009 for the first time in 35 years. As it is illegal in many areas of the world, potential medical uses are difficult to study.
In 2001 the United States Drug Enforcement Administration stated that LSD "produces no aphrodisiac effects, does not increase creativity, has no lasting positive effect in treating alcoholics or criminals, does not produce a "model psychosis", and does not generate immediate personality change." More recently, experimental uses of LSD have included the treatment of alcoholism, pain and cluster headache relief, and prospective studies on depression.
A 2020 meta-review indicated possible positive effects of LSD in reducing psychiatric symptoms, mainly in cases of alcoholism. There is evidence that psychedelics induce molecular and cellular adaptations related to neuroplasticity and that these could potentially underlie therapeutic benefits.
Psychedelic therapy
See also: Psychedelic therapyIn the 1950s and 1960s, LSD was used in psychiatry to enhance psychotherapy, known as psychedelic therapy. Some psychiatrists, such as Ronald A. Sandison, who pioneered its use at Powick Hospital in England, believed LSD was especially useful at helping patients to "unblock" repressed subconscious material through other psychotherapeutic methods, and also for treating alcoholism. One study concluded, "The root of the therapeutic value of the LSD experience is its potential for producing self-acceptance and self-surrender," presumably by forcing the user to face issues and problems in that individual's psyche.
Two recent reviews concluded that conclusions drawn from most of these early trials are unreliable due to serious methodological flaws. These include the absence of adequate control groups, lack of follow-up, and vague criteria for therapeutic outcome. In many cases, studies failed to convincingly demonstrate whether the drug or the therapeutic interaction was responsible for any beneficial effects.
In recent years, organizations like the Multidisciplinary Association for Psychedelic Studies (MAPS) have renewed clinical research of LSD.
It has been proposed that LSD be studied for use in the therapeutic setting, particularly in anxiety. In 2024, the FDA designated a form of LSD as a breakthrough therapy to treat generalized anxiety disorder which is being developed by MindMed.
Other uses
In the 1950s and 1960s, some psychiatrists (e.g., Oscar Janiger) explored the potential effect of LSD on creativity. Experimental studies attempted to measure the effect of LSD on creative activity and aesthetic appreciation. In 1966 Dr. James Fadiman conducted a study with the central question "How can psychedelics be used to facilitate problem solving?" This study attempted to solve 44 different problems and had 40 satisfactory solutions when the FDA banned all research into psychedelics. LSD was a key component of this study.
Since 2008 there has been ongoing research into using LSD to alleviate anxiety for terminally ill cancer patients coping with their impending deaths.
A 2012 meta-analysis found evidence that a single dose of LSD in conjunction with various alcoholism treatment programs was associated with a decrease in alcohol abuse, lasting for several months, but no effect was seen at one year. Adverse events included seizure, moderate confusion and agitation, nausea, vomiting, and acting in a bizarre fashion.
LSD has been used as a treatment for cluster headaches with positive results in some small studies.
LSD is a potent psychoplastogen, a compound capable of promoting rapid and sustained neural plasticity that may have wide-ranging therapeutic benefit. LSD has been shown to increase markers of neuroplasticity in human brain organoids and improve memory performance in human subjects.
LSD may have analgesic properties related to pain in terminally ill patients and phantom pain and may be useful for treating inflammatory diseases including rheumatoid arthritis.
Notable individuals
Some notable individuals have commented publicly on their experiences with LSD. Some of these comments date from the era when it was legally available in the US and Europe for non-medical uses, and others pertain to psychiatric treatment in the 1950s and 1960s. Still others describe experiences with illegal LSD, obtained for philosophic, artistic, therapeutic, spiritual, or recreational purposes.
- W. H. Auden, the poet, said, "I myself have taken mescaline once and L.S.D. once. Aside from a slight schizophrenic dissociation of the I from the Not-I, including my body, nothing happened at all." He also said, "LSD was a complete frost. … What it does seem to destroy is the power of communication. I have listened to tapes done by highly articulate people under LSD, for example, and they talk absolute drivel. They may have seen something interesting, but they certainly lose either the power or the wish to communicate." He also said, "Nothing much happened but I did get the distinct impression that some birds were trying to communicate with me."
- Daniel Ellsberg, an American peace activist, says he has had several hundred experiences with psychedelics.
- Richard Feynman, a notable physicist at California Institute of Technology, tried LSD during his professorship at Caltech. Feynman largely sidestepped the issue when dictating his anecdotes; he mentions it in passing in the "O Americano, Outra Vez" section.
- Jerry Garcia stated in a July 3, 1989 interview for Relix Magazine, in response to the question "Have your feelings about LSD changed over the years?," "They haven't changed much. My feelings about LSD are mixed. It's something that I both fear and that I love at the same time. I never take any psychedelic, have a psychedelic experience, without having that feeling of, "I don't know what's going to happen." In that sense, it's still fundamentally an enigma and a mystery."
- Bill Gates implied in an interview with Playboy that he tried LSD during his youth.
- Aldous Huxley, author of Brave New World, became a user of psychedelics after moving to Hollywood. He was at the forefront of the counterculture's use of psychedelic drugs, which led to his 1954 work The Doors of Perception. Dying from cancer, he asked his wife on 22 November 1963 to inject him with 100 μg of LSD. He died later that day.
- Steve Jobs, co-founder and former CEO of Apple Inc., said, "Taking LSD was a profound experience, one of the most important things in my life."
- Ernst Jünger, German writer and philosopher, throughout his life had experimented with drugs such as ether, cocaine, and hashish; and later in life he used mescaline and LSD. These experiments were recorded comprehensively in Annäherungen (1970, Approaches). The novel Besuch auf Godenholm (1952, Visit to Godenholm) is clearly influenced by his early experiments with mescaline and LSD. He met with LSD inventor Albert Hofmann and they took LSD together several times. Hofmann's memoir LSD, My Problem Child describes some of these meetings.
- In a 2004 interview, Paul McCartney said that The Beatles' songs "Day Tripper" and "Lucy in the Sky with Diamonds" were inspired by LSD trips. Nonetheless, John Lennon consistently stated over the course of many years that the fact that the initials of "Lucy in the Sky with Diamonds" spelled out L-S-D was a coincidence (he stated that the title came from a picture drawn by his son Julian) and that the band members did not notice until after the song had been released, and Paul McCartney corroborated that story. John Lennon, George Harrison, and Ringo Starr also used the drug, although McCartney cautioned that "it's easy to overestimate the influence of drugs on the Beatles' music."
- Michel Foucault had an LSD experience with Simeon Wade in Death Valley and later wrote "it was the greatest experience of his life, and that it profoundly changed his life and his work." According to Wade, as soon as he came back to Paris, Foucault scrapped the second History of Sexuality's manuscript, and totally rethought the whole project.
- Kary Mullis is reported to credit LSD with helping him develop DNA amplification technology, for which he received the Nobel Prize in Chemistry in 1993.
- Carlo Rovelli, an Italian theoretical physicist and writer, has credited his use of LSD with sparking his interest in theoretical physics.
- Oliver Sacks, a neurologist famous for writing best-selling case histories about his patients' disorders and unusual experiences, talks about his own experiences with LSD and other perception altering chemicals, in his book, Hallucinations.
- Matt Stone and Trey Parker, creators of the TV series South Park, claimed to have shown up at the 72nd Academy Awards, at which they were nominated for Best Original Song, under the influence of LSD.
See also
Notes
- The potency of N-benzylphenethylamines via buccal, sublingual, or nasal absorption is 50–100 greater (by weight) than oral route compared to the parent 2C-x compounds. Researches hypothesize the low oral metabolic stability of N-benzylphenethylamines is likely causing the low bioavailability on the oral route, although the metabolic profile of this compounds remains unpredictable; therefore researches state that the fatalities linked to these substances may partly be explained by differences in the metabolism between individuals.
References
- "Definition of "amide"". Collins English Dictionary. Archived from the original on April 2, 2015. Retrieved January 31, 2015.
- "American Heritage Dictionary Entry: amide". Ahdictionary.com. Archived from the original on April 2, 2015. Retrieved January 31, 2015.
- "amide – definition of amide in English from the Oxford Dictionary". Oxforddictionaries.com. Archived from the original on April 2, 2015. Retrieved January 31, 2015.
- Halpern JH, Suzuki J, Huertas PE, Passie T (June 7, 2014). "Hallucinogen Abuse and Dependence". In Price LH, Stolerman IP (eds.). Encyclopedia of Psychopharmacology A Springer Live Reference. Heidelberg, Germany: Springer-Verlag Berlin Heidelberg. pp. 1–5. doi:10.1007/978-3-642-27772-6_43-2. ISBN 978-3-642-27772-6.
Hallucinogen abuse and dependence are known complications resulting from ... LSD and psilocybin. Users do not experience withdrawal symptoms, but the general criteria for substance abuse and dependence otherwise apply. Dependence is estimated in approximately 2 % of recent-onset users
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 375. ISBN 9780071481274. Archived from the original on August 28, 2023. Retrieved June 12, 2023.
Several other classes of drugs are categorized as drugs of abuse but rarely produce compulsive use. These include psychedelic agents, such as lysergic acid diethylamide (LSD)
- ^ Dolder PC, Schmid Y, Haschke M, Rentsch KM, Liechti ME (June 2015). "Pharmacokinetics and Concentration-Effect Relationship of Oral LSD in Humans". The International Journal of Neuropsychopharmacology. 19 (1): pyv072. doi:10.1093/ijnp/pyv072. PMC 4772267. PMID 26108222.
- ^ Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A (2008). "The pharmacology of lysergic acid diethylamide: a review". CNS Neuroscience & Therapeutics. 14 (4): 295–314. doi:10.1111/j.1755-5949.2008.00059.x. PMC 6494066. PMID 19040555.
- Neinstein LS (2008). Adolescent Health Care: A Practical Guide. Lippincott Williams & Wilkins. p. 931. ISBN 9780781792561. Archived from the original on December 26, 2018. Retrieved January 27, 2017.
- ^ Mucke HA (July 2016). "From Psychiatry to Flower Power and Back Again: The Amazing Story of Lysergic Acid Diethylamide". Assay and Drug Development Technologies. 14 (5): 276–281. doi:10.1089/adt.2016.747. PMID 27392130.
- Kranzler HR, Ciraulo DA (April 2, 2007). Clinical Manual of Addiction Psychopharmacology. American Psychiatric Pub. p. 216. ISBN 9781585626632. Archived from the original on December 26, 2018. Retrieved January 27, 2017.
- "Lysergide". pubchem.ncbi.nlm.nih.gov. Archived from the original on April 12, 2023. Retrieved April 12, 2023.
- ^ Nichols DE (April 2016). Barker EL (ed.). "Psychedelics". Pharmacological Reviews. 68 (2): 264–355. doi:10.1124/pr.115.011478. ISSN 0031-6997. PMC 4813425. PMID 26841800.
- Liechti ME, Dolder PC, Schmid Y (May 2017). "Alterations of consciousness and mystical-type experiences after acute LSD in humans". Psychopharmacology. 234 (9–10): 1499–1510. doi:10.1007/s00213-016-4453-0. PMC 5420386. PMID 27714429.
- Griffiths RR, Hurwitz ES, Davis AK, Johnson MW, Jesse R (April 23, 2019). "Survey of subjective "God encounter experiences": Comparisons among naturally occurring experiences and those occasioned by the classic psychedelics psilocybin, LSD, ayahuasca, or DMT". PLOS ONE. 14 (4): e0214377. Bibcode:2019PLoSO..1414377G. doi:10.1371/journal.pone.0214377. PMC 6478303. PMID 31013281.
- Leptourgos P, Fortier-Davy M, Carhart-Harris R, Corlett PR, Dupuis D, Halberstadt AL, et al. (December 2020). "Hallucinations Under Psychedelics and in the Schizophrenia Spectrum: An Interdisciplinary and Multiscale Comparison". Schizophrenia Bulletin. 46 (6): 1396–1408. doi:10.1093/schbul/sbaa117. PMC 7707069. PMID 32944778.
- Holze F, Vizeli P, Ley L, Müller F, Dolder P, Stocker M, et al. (February 2021). "Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects". Neuropsychopharmacology. 46 (3): 537–544. doi:10.1038/s41386-020-00883-6. PMC 8027607. PMID 33059356.
- "LSD Fast Facts". www.justice.gov. Retrieved December 29, 2024.
- ^ "Commonly Abused Drugs Charts". National Institute on Drug Abuse. July 2, 2018. Archived from the original on March 1, 2020. Retrieved July 14, 2018.
- ^ Halpern JH, Lerner AG, Passie T (2018). A Review of Hallucinogen Persisting Perception Disorder (HPPD) and an Exploratory Study of Subjects Claiming Symptoms of HPPD. Current Topics in Behavioral Neurosciences. Vol. 36. pp. 333–360. doi:10.1007/7854_2016_457. ISBN 978-3-662-55878-2. PMID 27822679.
- ^ "LSD profile (chemistry, effects, other names, synthesis, mode of use, pharmacology, medical use, control status)". EMCDDA. Archived from the original on April 28, 2021. Retrieved July 14, 2018.
- Sloat S (January 27, 2017). "This is Why You Can't Escape an Hours-Long Acid Trip". Inverse. Archived from the original on June 11, 2021. Retrieved February 3, 2020.
- "What are hallucinogens?". National Institute of Drug Abuse. January 2016. Archived from the original on April 17, 2016. Retrieved April 24, 2016.
- Gershon L (July 19, 2016). "How LSD Went From Research to Religion". JSTOR Daily. Archived from the original on January 28, 2021. Retrieved July 14, 2018.
- ^ Nichols DE (February 2004). "Hallucinogens". Pharmacology & Therapeutics. 101 (2): 131–181. doi:10.1016/j.pharmthera.2003.11.002. ISSN 1879-016X. PMID 14761703.
- Girn M, Roseman L, Bernhardt B, Smallwood J, Carhart-Harris R, Spreng RN (May 3, 2020). "Serotonergic psychedelic drugs LSD and psilocybin reduce the hierarchical differentiation of unimodal and transmodal cortex". bioRxiv. doi:10.1101/2020.05.01.072314. S2CID 233346402.
- ^ Chwelos N, Blewett DB, Smith CM, Hoffer A (September 1959). "Use of d-lysergic acid diethylamide in the treatment of alcoholism". Quarterly Journal of Studies on Alcohol. 20 (3): 577–590. doi:10.15288/qjsa.1959.20.577. PMID 13810249.
- United States Congress House Committee on Interstate and Foreign Commerce Subcommittee on Public Health and Welfare (1968). Increased Controls Over Hallucinogens and Other Dangerous Drugs. U.S. Government Printing Office. Archived from the original on July 13, 2020. Retrieved August 3, 2021.
- "Psychiatric Research with Hallucinogens". www.druglibrary.org. Archived from the original on July 26, 2021. Retrieved July 26, 2021.
- ^ National Institute on Drug Abuse. "Hallucinogens". Archived from the original on June 3, 2020. Retrieved July 14, 2018.
- Yockey RA, Vidourek RA, King KA (July 2020). "Trends in LSD use among US adults: 2015–2018". Drug and Alcohol Dependence. 212: 108071. doi:10.1016/j.drugalcdep.2020.108071. PMID 32450479. S2CID 218893155.
- "DrugFacts: Hallucinogens – LSD, Peyote, Psilocybin, and PCP". National Institute on Drug Abuse. December 2014. Archived from the original on February 16, 2015. Retrieved February 17, 2015.
- Fahey D, Miller JS (eds.). Alcohol and Drugs in North America: A Historical Encyclopedia. p. 375. ISBN 978-1-59884-478-8.
- San Francisco Chronicle September 20, 1966 Page One
- Grof S, Grof JH (1979). Realms of the Human Unconscious (Observations from LSD Research). London: Souvenir Press (E & A) Ltd. pp. 13–14. ISBN 978-0-285-64882-1. Archived from the original on October 18, 2007. Retrieved November 18, 2007.
- ^ Nutt DJ, King LA, Nichols DE (August 2013). "Effects of Schedule I drug laws on neuroscience research and treatment innovation". Nature Reviews. Neuroscience. 14 (8): 577–585. doi:10.1038/nrn3530. PMID 23756634. S2CID 1956833.
- Campbell D (July 23, 2016). "Scientists study possible health benefits of LSD and ecstasy | Science". The Guardian. Archived from the original on July 23, 2016. Retrieved July 23, 2016.
- ^ Krebs TS, Johansen PØ (July 2012). "Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials". Journal of Psychopharmacology. 26 (7): 994–1002. doi:10.1177/0269881112439253. PMID 22406913. S2CID 10677273.
- ^ Lustberg D (October 14, 2022). "Acid for Anxiety: Fast and Lasting Anxiolytic Effects of LSD". Psychedelic Science Review. Archived from the original on December 1, 2022. Retrieved December 1, 2022.
- ^ Holze F, Gasser P, Müller F, Dolder PC, Liechti ME (September 2022). "Lysergic Acid Diethylamide-Assisted Therapy in Patients With Anxiety With and Without a Life-Threatening Illness: A Randomized, Double-Blind, Placebo-Controlled Phase II Study". Biological Psychiatry. 93 (3): 215–223. doi:10.1016/j.biopsych.2022.08.025. PMID 36266118. S2CID 252095586.
- Dos Santos RG, Osório FL, Crippa JA, Riba J, Zuardi AW, Hallak JE (June 2016). "Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years". Therapeutic Advances in Psychopharmacology. 6 (3): 193–213. doi:10.1177/2045125316638008. PMC 4910400. PMID 27354908.
- "History of LSD Therapy". druglibrary.org. Archived from the original on November 7, 2022. Retrieved November 7, 2022.
- "Hallucinogens – LSD, Peyote, Psilocybin, and PCP". NIDA InfoFacts. The National Institute on Drug Abuse (NIDA). June 2009. Archived from the original on November 21, 2009.
- Schiff PL (October 2006). "Ergot and its alkaloids". American Journal of Pharmaceutical Education. 70 (5): 98. doi:10.5688/aj700598 (inactive November 19, 2024). PMC 1637017. PMID 17149427.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - El-Mallakh RS, Walker KL (2010). "Hallucinations, psuedohallucinations, and parahallucinations". Psychiatry. 73 (1): 34–42. doi:10.1521/psyc.2010.73.1.34. PMID 20235616.
- "LSD Fast Facts". www.justice.gov. Retrieved December 29, 2024.
- Majić T, Schmidt TT, Gallinat J (March 2015). "Peak experiences and the afterglow phenomenon: when and how do therapeutic effects of hallucinogens depend on psychedelic experiences?". Journal of Psychopharmacology. 29 (3): 241–253. doi:10.1177/0269881114568040. PMID 25670401. S2CID 16483172.
- ^ Honig D. "Frequently Asked Questions". Erowid. Archived from the original on February 12, 2016.
- ^ McGlothlin W, Cohen S, McGlothlin MS (November 1967). "Long lasting effects of LSD on normals" (PDF). Archives of General Psychiatry. 17 (5): 521–532. doi:10.1001/archpsyc.1967.01730290009002. PMID 6054248. Archived from the original (PDF) on April 30, 2011.
- Kopra EI, Ferris JA, Rucker JJ, McClure B, Young AH, Copeland CS, et al. (August 2022). "Adverse experiences resulting in emergency medical treatment seeking following the use of lysergic acid diethylamide (LSD)". Journal of Psychopharmacology. 36 (8): 956–964. doi:10.1177/02698811221099650. PMC 9353972. PMID 35672900.
- Rogge T (May 21, 2014), Substance use – LSD, MedlinePlus, U.S. National Library of Medicine, archived from the original on July 28, 2016, retrieved July 14, 2016
- CESAR (October 29, 2013), LSD, Center for Substance Abuse Research, University of Maryland, archived from the original on July 15, 2016, retrieved July 14, 2016
- ^ Linton HR, Langs RJ (May 1962). "Subjective Reactions to Lysergic Acid Diethylamide (LSD-25)". Archives of General Psychiatry. 6 (5): 352–368. doi:10.1001/archpsyc.1962.01710230020003.
- Katz MM, Waskow IE, Olsson J (February 1968). "Characterizing the psychological state produced by LSD". Journal of Abnormal Psychology. 73 (1): 1–14. CiteSeerX 10.1.1.409.4030. doi:10.1037/h0020114. PMID 5639999.
- Parker LA (June 1996). "LSD produces place preference and flavor avoidance but does not produce flavor aversion in rats". Behavioral Neuroscience. 110 (3): 503–508. doi:10.1037/0735-7044.110.3.503. PMID 8888996.
- Oster G (1966). "Moiré patterns and visual hallucinations" (PDF). Psychedelic Review. 7: 33–40. Archived (PDF) from the original on April 19, 2017.
- Kaelen M, Roseman L, Kahan J, Santos-Ribeiro A, Orban C, Lorenz R, et al. (July 2016). "LSD modulates music-induced imagery via changes in parahippocampal connectivity". European Neuropsychopharmacology. 26 (7): 1099–1109. doi:10.1016/j.euroneuro.2016.03.018. PMID 27084302. S2CID 24037275.
- Nutt DJ, King LA, Phillips LD (November 2010). "Drug harms in the UK: a multicriteria decision analysis". Lancet. 376 (9752): 1558–1565. CiteSeerX 10.1.1.690.1283. doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. S2CID 5667719.
- Nutt D, King LA, Saulsbury W, Blakemore C (March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". Lancet. 369 (9566): 1047–53. doi:10.1016/s0140-6736(07)60464-4. PMID 17382831. S2CID 5903121.
- ^ Nichols DE, Grob CS (March 2018). "Is LSD Toxic?". Forensic Science International. 284: 141–145. doi:10.1016/j.forsciint.2018.01.006. PMID 29408722.
- Nutt DJ, King LA, Phillips LD (November 2010). "Drug harms in the UK: a multicriteria decision analysis". Lancet. 376 (9752): 1558–65. CiteSeerX 10.1.1.690.1283. doi:10.1016/s0140-6736(10)61462-6. PMID 21036393. S2CID 5667719.
- Krebs TS, Johansen PØ (August 19, 2013). Lu L (ed.). "Psychedelics and mental health: a population study". PLOS ONE. 8 (8): e63972. Bibcode:2013PLoSO...863972K. doi:10.1371/journal.pone.0063972. PMC 3747247. PMID 23976938.
- Murray RM, Paparelli A, Morrison PD, Marconi A, Di Forti M (October 2013), "What can we learn about schizophrenia from studying the human model, drug-induced psychosis?", American Journal of Medical Genetics Part B, Special Issue: Identifying the Origins of Mental Illness: A Festschrift in Honor of Ming T. Tsuang, 162 (7): 661–670, doi:10.1002/ajmg.b.32177, PMID 24132898, S2CID 205326399
- Rockefeller IV JD (December 8, 1994). "Is Military Research Hazardous to Veterans Health? Lessons Spanning Half A Century, part F. HALLUCINOGENS". West Virginia: 103rd Congress, 2nd Session-S. Prt. 103-97; Staff Report prepared for the committee on veterans' affairs. Archived from the original on August 13, 2006. Retrieved December 13, 2018.
- Middlefell R (March 1967). "The effects of LSD on body sway suggestibility in a group of hospital patients" (PDF). The British Journal of Psychiatry. 113 (496): 277–280. doi:10.1192/bjp.113.496.277. PMID 6029626. S2CID 19439549. Archived from the original (PDF) on April 30, 2011.
- Sjoberg BM, Hollister LE (November 1965). "The effects of psychotomimetic drugs on primary suggestibility". Psychopharmacologia. 8 (4): 251–262. doi:10.1007/BF00407857. PMID 5885648. S2CID 15249061.
- Halpern JH, Pope HG (March 2003). "Hallucinogen persisting perception disorder: what do we know after 50 years?". Drug and Alcohol Dependence. 69 (2): 109–19. doi:10.1016/S0376-8716(02)00306-X. PMID 12609692.
- Müller F, Kraus E, Holze F, Becker A, Ley L, Schmid Y, et al. (January 2022). "Flashback phenomena after administration of LSD and psilocybin in controlled studies with healthy participants". Psychopharmacology. 239 (6): 1933–1943. doi:10.1007/s00213-022-06066-z. PMC 9166883. PMID 35076721. S2CID 246276633.
- Johansen PØ, Krebs TS (March 2015). "Psychedelics not linked to mental health problems or suicidal behavior: a population study". Journal of Psychopharmacology. 29 (3): 270–279. doi:10.1177/0269881114568039. PMID 25744618. S2CID 2025731.
- Holland D, Passie T (2011). Flashback-Phänomene als Nachwirkung von Halluzinogeneinnahme. Bewusstsein – Kognition – Erleben (in German). Vol. 2. VWB Report. ISBN 978-3-86135-207-5. Archived from the original on June 9, 2023. Retrieved June 9, 2023.
- Abraham HD, Duffy FH (October 1996). "Stable quantitative EEG difference in post-LSD visual disorder by split-half analysis: evidence for disinhibition". Psychiatry Research. 67 (3): 173–87. doi:10.1016/0925-4927(96)02833-8. PMID 8912957. S2CID 7587687.
- ^ Nutt DJ, Castle D (March 7, 2023). "Drug-interaction with psychotropic drugs". Psychedelics as Psychiatric Medications. Oxford University Press. ISBN 9780192678522. Archived from the original on May 21, 2023. Retrieved May 21, 2023.
- Simonsson O, Goldberg SB, Chambers R, Osika W, Long DM, Hendricks PS (October 1, 2022). "Prevalence and associations of classic psychedelic-related seizures in a population-based sample". Drug and Alcohol Dependence. 239. 109586. doi:10.1016/j.drugalcdep.2022.109586. PMC 9627432. PMID 35981469.
- Fisher D, Ungerleider J (1967). "Grand mal seizures following ingestion of LSD". Western Journal of Medicine. 106 (3): 201–211. PMC 1502729. PMID 4962683.
- Buchborn T, Grecksch G, Dieterich D, Hollt V (2016). "Chapter 79 - Tolerance to Lysergic Acid Diethylamide: Overview, Correlates, and Clinical Implications". Neuropathology of Drug Addictions and Substance Misuse. Vol. 2. Academic Press. pp. 848–849. doi:10.1016/B978-0-12-800212-4.00079-0. ISBN 978-0-12-800212-4.
- ^ Dolder DS, Grünblatt E, Müller F, Borgwardt SJ, Liechti ME (June 28, 2017). "A Single Dose of LSD Does Not Alter Gene Expression of the Serotonin 2A Receptor Gene (HTR2A) or Early Growth Response Genes (EGR1-3) in Healthy Subjects". Frontiers in Neuroscience. 8: 423. doi:10.3389/fphar.2017.00423. PMC 5487530. PMID 28701958.
- Kooijman NI, Willegers T, Reuser A, Mulleners WM, Kramers C, Vissers KCP, et al. (January 4, 2023). "Are psychedelics the answer to chronic pain: A review of current literature". Pain Practice. 23 (4): 455. doi:10.1111/papr.13203. hdl:2066/291903. ISSN 1533-2500. PMID 36597700. S2CID 255470638.
- Wolbach AB, Isbell H, Miner EJ (March 1962). "Cross tolerance between mescaline and LSD-25, with a comparison of the mescaline and LSD reactions". Psychopharmacologia. 3: 1–14. doi:10.1007/BF00413101. PMID 14007904. S2CID 23803624. Archived from the original on April 19, 2014. Retrieved December 1, 2007.
- Isbell H, Wolbach AB, Wikler A, Miner EJ (1961). "Cross tolerance between LSD and psilocybin". Psychopharmacologia. 2 (3): 147–159. doi:10.1007/BF00407974. PMID 13717955. S2CID 7746880. Archived from the original on March 15, 2016. Retrieved December 1, 2007.
- Rosenberg D, Isbell H, Miner E, Logan C (August 7, 1963). "The effect of N,N-dimethyltryptamine in human subjects tolerant to lysergic acid diethylamide". Psychopharmacologia. 5 (3): 223–224. doi:10.1007/BF00413244. PMID 14138757. S2CID 32950588.
- Jonas S, Downer JD (October 1964). "Gross behavioural changes in monkeys following administration of LSD-25, and development of tolerance to LSD-25". Psychopharmacologia. 6 (4): 303–386. doi:10.1007/BF00413161. PMID 4953438. S2CID 11768927.
- Schlemmer RF, Nawara C, Heinze WJ, Davis JM, Advokat C (March 1986). "Influence of environmental context on tolerance to LSD-induced behavior in primates". Biological Psychiatry. 21 (3): 314–317. doi:10.1016/0006-3223(86)90053-3. PMID 3947713. S2CID 35508993.
- Lüscher C, Ungless MA (November 2006). "The mechanistic classification of addictive drugs". PLOS Medicine. 3 (11): e437. doi:10.1371/journal.pmed.0030437. PMC 1635740. PMID 17105338.
- ^ Liechti ME (October 2017). "Modern Clinical Research on LSD". Neuropsychopharmacology. 42 (11): 2114–2127. doi:10.1038/npp.2017.86. PMC 5603820. PMID 28447622.
- Balestrieri A, Fontanari D (September 1959). "Acquired and crossed tolerance to mescaline, LSD-25, and BOL-148". Archives of General Psychiatry. 1 (3): 279–282. doi:10.1001/archpsyc.1959.03590030063008. PMID 13796178.
- Hamill J, Hallak J, Dursun SD, Baker G (2019). "Ayahuasca: Psychological and Physiologic Effects, Pharmacology and Potential Uses in Addiction and Mental Illness". Current Neuropharmacology. 17 (2): 1–15. doi:10.2174/1570159X16666180125095902. ISSN 1875-6190. PMC 6343205. PMID 29366418.
- Morgenstern J, Langenbucher J, Labouvie E (September 1994). "The generalizability of the dependence syndrome across substances: an examination of some properties of the proposed DSM-IV dependence criteria". Addiction. 89 (9). Society for the Study of Addiction: 1105–1113. doi:10.1111/j.1360-0443.1994.tb02787.x. PMID 7987187.
- Li JH, Lin LF (November 1998). "Genetic toxicology of abused drugs: a brief review". Mutagenesis. 13 (6): 557–65. doi:10.1093/mutage/13.6.557. PMID 9862186.
- ^ Lipow M, Kaleem SZ, Espiridion E (March 2022). "NBOMe Toxicity and Fatalities: A Review of the Literature". Transformative Medicine. 1 (1): 12–18. doi:10.54299/tmed/msot8578. ISSN 2831-8978. S2CID 247888583.
- Klock JC, Boerner U, Becker CE (March 1974). "Coma, Hyperthermia and Bleeding Associated with Massive LSD Overdose: A Report of Eight Cases". The Western Journal of Medicine. 120 (3): 183–188. PMC 1129381. PMID 4816396.
- ^ LSD Toxicity Treatment & Management~treatment at eMedicine
- Zawilska JB, Kacela M, Adamowicz P (February 26, 2020). "NBOMes–Highly Potent and Toxic Alternatives of LSD". Frontiers in Neuroscience. 14: 78. doi:10.3389/fnins.2020.00078. PMC 7054380. PMID 32174803.
- Hartogsohn I (2017). "Constructing drug effects: A history of set and setting". Drug Science, Policy and Law. 3: 205032451668332. doi:10.1177/2050324516683325. ISSN 2050-3245. S2CID 53373205.
- ^ Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Johnson RA, Janowsky A (December 2018). "Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors". Biochemical Pharmacology. 158: 27–34. doi:10.1016/j.bcp.2018.09.024. PMC 6298744. PMID 30261175.
- Poklis JL, Raso SA, Alford KN, Poklis A, Peace MR (October 2015). "Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and Other Dimethoxyphenyl-N-[(2-Methoxyphenyl) Methyl]Ethanamine Derivatives on Blotter Paper". Journal of Analytical Toxicology. 39 (8): 617–623. doi:10.1093/jat/bkv073. PMC 4570937. PMID 26378135.
- ^ Ivory ST, Rotella J, Schumann J, Greene SL (March 28, 2022). "A cluster of 25B-NBOH poisonings following exposure to powder sold as lysergic acid diethylamide (LSD)". Clinical Toxicology. 60 (8): 966–969. doi:10.1080/15563650.2022.2053150. PMID 35343858. S2CID 247764056.
- Miliano C, Marti M, Pintori N, Castelli MP, Tirri M, Arfè R, et al. (December 12, 2019). "Neurochemical and Behavioral Profiling in Male and Female Rats of the Psychedelic Agent 25I-NBOMe". Frontiers in Pharmacology. 10: 1406. doi:10.3389/fphar.2019.01406. PMC 6921684. PMID 31915427.
- ^ Leth-Petersen S, Bundgaard C, Hansen M, Carnerup MA, Kehler J, Kristensen JL (February 14, 2014). "Correlating the Metabolic Stability of Psychedelic 5-HT2A Agonists with Anecdotal Reports of Human Oral Bioavailability". Neurochemical Research. 39 (10): 2018–2023. doi:10.1007/s11064-014-1253-y. PMID 24519542. S2CID 254857910.
- Halberstadt AL (January 18, 2017). "Pharmacology and Toxicology of N-Benzylphenethylamine ("NBOMe") Hallucinogens". Neuropharmacology of New Psychoactive Substances. Current Topics in Behavioral Neurosciences. Vol. 32. Springer. pp. 283–311. doi:10.1007/7854_2016_64. ISBN 978-3-319-52444-3. PMID 28097528.
- Duffau B, Camargo C, Kogan M, Fuentes E, Kennedy Cassels B (August 2016). "Analysis of 25 C NBOMe in Seized Blotters by HPTLC and GC–MS". Journal of Chromatographic Science. 54 (7): 1153–1158. doi:10.1093/chromsci/bmw095. PMC 4941995. PMID 27406128.
- Francesco SB, Ornella C, Gabriella A, Giuseppe V, Rita S, Flaminia BP, et al. (July 3, 2014). "25C-NBOMe: preliminary data on pharmacology, psychoactive effects, and toxicity of a new potent and dangerous hallucinogenic drug". BioMed Research International. 2014: 734749. doi:10.1155/2014/734749. PMC 4106087. PMID 25105138.
- Potts AJ, Thomas SHL, Hill SL (September 2021). "Pharmacology and toxicology of N-Benzyl-phenylethylamines (25X-NBOMe) hallucinogens". In Dargan P, Wood D (eds.). Novel Psychoactive Substances: Classification, Pharmacology and Toxicology (2nd ed.). Academic Press. pp. 279–300. doi:10.1016/B978-0-12-818788-3.00008-5. ISBN 978-0-12-818788-3. S2CID 240583877.
- Díaz Moreno M, Alarcón Ayala N, Estrada Y, Morris V, Quintero J (November 2022). "Échele Cabeza as a harm reduction project and activist movement in Colombia". Drugs, Habits and Social Policy. 23 (3): 263–276. doi:10.1108/DHS-07-2022-0026. ISSN 2752-6739.
- Clancy L, Philp M, Shimmon R, Fu S (May 2021). "Development and validation of a color spot test method for the presumptive detection of 25-NBOMe compounds". Drug Testing and Analysis. 13 (5): 929–943. doi:10.1002/dta.2905. PMID 32744773.
- ^ "PDSP Database". UNC (in Zulu). Retrieved December 11, 2024.
- Liu T. "BindingDB BDBM21342 (4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[7.6.1.0^{2,7}.0^{12,16}]hexadeca-1(16),2,9,12,14-pentaene-4-carboxamide::CHEMBL263881::LSD::LSD 25::LSD,(+)::LSD,l-::Lysergic Acid Diethylamide::Lysergic Acid Diethylamide Tartrate::US20240166618, Compound LSD::[3H]-LSD::d-Isolysergic acid amide". BindingDB. Retrieved December 11, 2024.
- Holze F, Singh N, Liechti ME, D'Souza DC (May 2024). "Serotonergic Psychedelics: A Comparative Review of Efficacy, Safety, Pharmacokinetics, and Binding Profile". Biol Psychiatry Cogn Neurosci Neuroimaging. 9 (5): 472–489. doi:10.1016/j.bpsc.2024.01.007. PMID 38301886.
- Ray TS (February 2010). "Psychedelics and the human receptorome". PLOS ONE. 5 (2): e9019. Bibcode:2010PLoSO...5.9019R. doi:10.1371/journal.pone.0009019. PMC 2814854. PMID 20126400.
- Rickli A, Luethi D, Reinisch J, Buchy D, Hoener MC, Liechti ME (December 2015). "Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs)" (PDF). Neuropharmacology. 99: 546–553. doi:10.1016/j.neuropharm.2015.08.034. PMID 26318099.
- Rickli A, Moning OD, Hoener MC, Liechti ME (August 2016). "Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens" (PDF). Eur Neuropsychopharmacol. 26 (8): 1327–1337. doi:10.1016/j.euroneuro.2016.05.001. PMID 27216487.
- Luethi D, Trachsel D, Hoener MC, Liechti ME (May 2018). "Monoamine receptor interaction profiles of 4-thio-substituted phenethylamines (2C-T drugs)" (PDF). Neuropharmacology. 134 (Pt A): 141–148. doi:10.1016/j.neuropharm.2017.07.012. PMID 28720478.
- Janowsky A, Eshleman AJ, Johnson RA, Wolfrum KM, Hinrichs DJ, Yang J, et al. (July 2014). "Mefloquine and psychotomimetics share neurotransmitter receptor and transporter interactions in vitro". Psychopharmacology (Berl). 231 (14): 2771–2783. doi:10.1007/s00213-014-3446-0. PMC 4097020. PMID 24488404.
- Wsół A (December 2023). "Cardiovascular safety of psychedelic medicine: current status and future directions". Pharmacol Rep. 75 (6): 1362–1380. doi:10.1007/s43440-023-00539-4. PMC 10661823. PMID 37874530.
- Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, et al. (February 2000). "Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT(2A) and 5-HT(2C) receptors". Synapse. 35 (2): 144–150. doi:10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K. PMID 10611640.
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, et al. (September 1999). "Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells". Br J Pharmacol. 128 (1): 13–20. doi:10.1038/sj.bjp.0702751. PMC 1571597. PMID 10498829.
- Gainetdinov RR, Hoener MC, Berry MD (July 2018). "Trace Amines and Their Receptors". Pharmacol Rev. 70 (3): 549–620. doi:10.1124/pr.117.015305. PMID 29941461.
- Simmler LD, Buchy D, Chaboz S, Hoener MC, Liechti ME (April 2016). "In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1". J Pharmacol Exp Ther. 357 (1): 134–144. doi:10.1124/jpet.115.229765. PMID 26791601.
- Marona-Lewicka D, Thisted RA, Nichols DE (July 2005). "Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis". Psychopharmacology. 180 (3): 427–35. doi:10.1007/s00213-005-2183-9. PMID 15723230. S2CID 23565306.
- ^ Aghajanian GK, Bing OH (1964). "Persistence of lysergic acid diethylamide in the plasma of human subjects" (PDF). Clinical Pharmacology and Therapeutics. 5 (5): 611–614. doi:10.1002/cpt196455611. PMID 14209776. S2CID 29438767. Archived from the original (PDF) on March 27, 2009.
- Nelson DL (February 2004). "5-HT5 receptors". Current Drug Targets. CNS and Neurological Disorders. 3 (1): 53–58. doi:10.2174/1568007043482606. PMID 14965244.
- Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J (April 2011). "Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists". Neuroscience Letters. 493 (3): 76–79. doi:10.1016/j.neulet.2011.01.046. PMC 3064746. PMID 21276828.
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, et al. (January 2007). "Functional selectivity and classical concepts of quantitative pharmacology". The Journal of Pharmacology and Experimental Therapeutics. 320 (1): 1–13. doi:10.1124/jpet.106.104463. PMID 16803859. S2CID 447937. Archived from the original on June 11, 2023. Retrieved June 11, 2023.
- Aghajanian GK, Marek GJ (August 1999). "Serotonin and hallucinogens". Neuropsychopharmacology. 21 (2 Suppl): 16S – 23S. doi:10.1016/S0893-133X(98)00135-3. PMID 10432484.
- Svenningsson P, Nairn AC, Greengard P (October 2005). "DARPP-32 mediates the actions of multiple drugs of abuse". The AAPS Journal. 7 (2): E353-60. doi:10.1208/aapsj070235. PMC 2750972. PMID 16353915.
- ^ Borroto-Escuela DO, Romero-Fernandez W, Narvaez M, Oflijan J, Agnati LF, Fuxe K (January 2014). "Hallucinogenic 5-HT2AR agonists LSD and DOI enhance dopamine D2R protomer recognition and signaling of D2-5-HT2A heteroreceptor complexes". Biochemical and Biophysical Research Communications. 443 (1): 278–84. doi:10.1016/j.bbrc.2013.11.104. PMID 24309097.
- Green JP, Johnson CL, Weinstein H, Maayani S (December 1977). "Antagonism of histamine-activated adenylate cyclase in brain by D-lysergic acid diethylamide". Proceedings of the National Academy of Sciences of the United States of America. 74 (12): 5697–701. Bibcode:1977PNAS...74.5697G. doi:10.1073/pnas.74.12.5697. PMC 431860. PMID 23536.
- ^ Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM (September 2002). "Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD)". Journal of Medicinal Chemistry. 45 (19): 4344–9. doi:10.1021/jm020153s. PMID 12213075.
- ^ Chen Q, Tesmer JJ (January 2017). "A Receptor on Acid". Cell. 168 (3): 339–341. doi:10.1016/j.cell.2017.01.012. PMC 5520807. PMID 28129534.
- ^ Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, et al. (January 2017). "Crystal Structure of an LSD-Bound Human Serotonin Receptor". Cell. 168 (3): 377–389.e12. doi:10.1016/j.cell.2016.12.033. PMC 5289311. PMID 28129538.
- Calder AE, Hasler G (January 2023). "Towards an understanding of psychedelic-induced neuroplasticity". Neuropsychopharmacology. 48 (1): 104–112. doi:10.1038/s41386-022-01389-z. PMC 9700802. PMID 36123427.
- Moliner R, Girych M, Brunello CA, Kovaleva V, Biojone C, Enkavi G, et al. (June 2023). "Psychedelics promote plasticity by directly binding to BDNF receptor TrkB". Nature Neuroscience. 26 (6): 1032–1041. doi:10.1038/s41593-023-01316-5. PMC 10244169. PMID 37280397.
- ^ Carhart-Harris RL, Muthukumaraswamy S, Roseman L, Kaelen M, Droog W, Murphy K, et al. (April 11, 2016). "Neural correlates of the LSD experience revealed by multimodal neuroimaging". Proceedings of the National Academy of Sciences of the United States of America. 113 (17): 4853–4858. Bibcode:2016PNAS..113.4853C. doi:10.1073/pnas.1518377113. PMC 4855588. PMID 27071089.
- Singleton SP, Luppi AI, Carhart-Harris RL, Cruzat J, Roseman L, Nutt DJ, et al. (October 2022). "Receptor-informed network control theory links LSD and psilocybin to a flattening of the brain's control energy landscape". Nat Commun. 13 (1): 5812. Bibcode:2022NatCo..13.5812S. doi:10.1038/s41467-022-33578-1. PMC 9530221. PMID 36192411.
- Delli Pizzi S, Chiacchiaretta P, Sestieri C, Ferretti A, Onofrj M, Della Penna S, et al. (July 2023). "Spatial Correspondence of LSD-Induced Variations on Brain Functioning at Rest With Serotonin Receptor Expression". Biol Psychiatry Cogn Neurosci Neuroimaging. 8 (7): 768–776. doi:10.1016/j.bpsc.2023.03.009. PMID 37003409. S2CID 257862535.
- ^ Delli Pizzi S, Chiacchiaretta P, Sestieri C, Ferretti A, Tullo MG, Della Penna S, et al. (December 2023). "LSD-induced changes in the functional connectivity of distinct thalamic nuclei". NeuroImage. 283: 120414. doi:10.1016/j.neuroimage.2023.120414. PMID 37858906.
- ^ Shulgin A, Shulgin A (1997). "LSD". TiHKAL. Berkeley, CA: Transform Press. ISBN 0-9630096-9-9. Archived from the original on October 15, 2008.
- Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A (November 11, 2008). "The pharmacology of lysergic acid diethylamide: a review". CNS Neuroscience & Therapeutics. 14 (4): 295–314. doi:10.1111/j.1755-5949.2008.00059.x. PMC 6494066. PMID 19040555.
- Papac DI, Foltz RL (May–June 1990). "Measurement of lysergic acid diethylamide (LSD) in human plasma by gas chromatography/negative ion chemical ionization mass spectrometry" (PDF). Journal of Analytical Toxicology. 14 (3): 189–190. doi:10.1093/jat/14.3.189. PMID 2374410. Archived from the original on April 29, 2011.
- "LSD" (PDF). Handbook of Medical Hallucinogens. Guilford Publications. 2021. p. 160. ISBN 9781462545452. Archived (PDF) from the original on March 14, 2024. Retrieved March 14, 2024.
- Monte AP, Marona-Lewicka D, Kanthasamy A, Sanders-Bush E, Nichols DE (March 1995). "Stereoselective LSD-like activity in a series of d-lysergic acid amides of (R)- and (S)-2-aminoalkanes". Journal of Medicinal Chemistry. 38 (6): 958–66. doi:10.1021/jm00006a015. PMID 7699712.
- Kornfeld EC, Fornefeld EJ, Kline GB, Mann MJ, Morrison DE, Jones RG, et al. (1956). "The Total Synthesis of Lysergic Acid". Journal of the American Chemical Society. 78 (13): 3087–3114. Bibcode:1956JAChS..78.3087K. doi:10.1021/ja01594a039.
- Inuki S, Oishi S, Fujii N, Ohno H (November 2008). "Total synthesis of (+/-)-lysergic acid, lysergol, and isolysergol by palladium-catalyzed domino cyclization of amino allenes bearing a bromoindolyl group". Organic Letters. 10 (22): 5239–42. doi:10.1021/ol8022648. PMID 18956869.
- Nichols DE (2018). "Dark classics in chemical neuroscience: lysergic acid diethylamide (LSD)" (PDF). ACS Chemical Neuroscience. 9 (10): 2331–2343. doi:10.1021/acschemneuro.8b00403. Retrieved January 5, 2025.
- National University of Singapore, Yong Loo Lin School of Medicine (February 10, 2022). "Harvesting baker's yeast for aging-related therapeutics". ScienceDaily. Archived from the original on November 27, 2022. Retrieved May 4, 2023. Journal Reference: Wong G, Lim LR, Tan YQ, Go MK, Bell DJ, Freemont PS, et al. (February 2022). "Reconstituting the complete biosynthesis of D-lysergic acid in yeast". Nature Communications. 13 (1): 712. Bibcode:2022NatCo..13..712W. doi:10.1038/s41467-022-28386-6. PMC 8821704. PMID 35132076.
- Greiner T, Burch NR, Edelberg R (February 1958). "Psychopathology and psychophysiology of minimal LSD-25 dosage; a preliminary dosage-response spectrum". A.M.A. Archives of Neurology and Psychiatry. 79 (2): 208–10. doi:10.1001/archneurpsyc.1958.02340020088016. PMID 13497365.
- Meyer MA (April 2003). "Neurologic complications of anthrax: a review of the literature". Archives of Neurology. 60 (4). Schweiz: 483–8. doi:10.1001/archneur.60.4.483. PMID 12707059.
- Polito V, Stevenson RJ (February 6, 2019). "A systematic study of microdosing psychedelics". PLOS ONE. 14 (2): e0211023. Bibcode:2019PLoSO..1411023P. doi:10.1371/journal.pone.0211023. PMC 6364961. PMID 30726251.
- ^ Hidalgo E (2009). "LSD Samples Analysis". Erowid. Archived from the original on February 13, 2010. Retrieved February 8, 2010.
- ^ Henderson LA, Glass WJ (1994). LSD: Still with us after all these years. San Francisco: Jossey-Bass. ISBN 978-0-7879-4379-0.
- Fire & Earth Erowid (November 2003). "LSD Analysis – Do we know what's in street acid?". Erowid. Archived from the original on January 26, 2010. Retrieved February 8, 2010.
- Li Z, McNally AJ, Wang H, Salamone SJ (October 1998). "Stability study of LSD under various storage conditions". Journal of Analytical Toxicology. 22 (6): 520–5. doi:10.1093/jat/22.6.520. PMID 9788528.
- "Lysergide (LSD) drug profile". European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Archived from the original on February 2, 2023. Retrieved May 15, 2023.
- ^ Appel JB, Whitehead WE, Freedman DX (July 1968). "Motivation and the behavioral effects of LSD". Psychonomic Science. 12 (7): 305–306. doi:10.3758/BF03331322. ISSN 0033-3131. S2CID 144527673.
- R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 12th edition, Biomedical Publications, Foster City, CA, 2020, pp. 1197–1199.
- Dolder PC, Schmid Y, Steuer AE, Kraemer T, Rentsch KM, Hammann F, et al. (October 2017). "Pharmacokinetics and Pharmacodynamics of Lysergic Acid Diethylamide in Healthy Subjects". Clinical Pharmacokinetics. 56 (10): 1219–1230. doi:10.1007/s40262-017-0513-9. PMC 5591798. PMID 28197931.
- ^ Jiaming Z, Xin W, Jiali Z, Hang R, Yunli Z, Ping X (March 2023). "Concentrations of LSD, 2-oxo-3-hydroxy-LSD, and iso-LSD in hair segments of 18 drug abusers". Forensic Science International. 344. doi:10.1016/j.forsciint.2023.111578. PMID 36753839. S2CID 256574276.
- ^ Hofmann A (1980). LSD—My Problem Child. McGraw-Hill. ISBN 0-07-029325-2. Archived from the original on December 15, 2017. Retrieved April 19, 2010 – via The Psychedelic Library.
- ^ Hofmann A (2009). LSD, my problem child: reflections on sacred drugs, mysticism, and science (4th ed.). Santa Cruz, CA: Multidisciplinary Association for Psychedelic Studies. ISBN 978-0-9798622-2-9. OCLC 610059315.
- ^ Lee MA, Shlain B (1992). Acid dreams: the complete social history of LSD: the CIA, the Sixties, and beyond. New York: Grove Weidenfeld. ISBN 0-8021-3062-3. OCLC 25281992.
- Ettinger RH (2017). Psychopharmacology. Psychology Press. p. 226. ISBN 978-1-351-97870-5. Archived from the original on September 27, 2021. Retrieved September 27, 2021.
- Freedman AM, Ebin EV, Wilson EA (March 1962). "Autistic schizophrenic children. An experiment in the use of d-lysergic acid diethylamide (LSD-25)". Archives of General Psychiatry. 6 (3): 203–213. doi:10.1001/archpsyc.1962.01710210019003. PMID 13894863.
- Simmons JQ, Leiken SJ, Lovaas OI, Schaeffer B, Perloff B (May 1966). "Modification of autistic behavior with LSD-25". The American Journal of Psychiatry. 122 (11): 1201–1211. doi:10.1176/ajp.122.11.1201. PMID 5325567.
- Sigafoos J, Green VA, Edrisinha C, Lancioni GE (2007). "Flashback to the 1960s: LSD in the treatment of autism". Developmental Neurorehabilitation. 10 (1): 75–81. doi:10.1080/13638490601106277. PMID 17608329.
- Abramson HA (December 1967). "The use of LSD (d-lysergic acid diethylamide) in the therapy of children (a brief review)". The Journal of Asthma Research. 5 (2): 139–143. doi:10.3109/02770906709104325. PMID 4865578.
- "Psychiatric Research with Hallucinogens". www.druglibrary.org. Archived from the original on July 26, 2021. Retrieved July 26, 2021.
- United States Congress House Committee on Interstate and Foreign Commerce Subcommittee on Public Health and Welfare (1968). Increased Controls Over Hallucinogens and Other Dangerous Drugs. U.S. Government Printing Office. Archived from the original on July 13, 2020. Retrieved August 3, 2021.
- Yockey RA, Vidourek RA, King KA (July 2020). "Trends in LSD use among US adults: 2015–2018". Drug and Alcohol Dependence. 212: 108071. doi:10.1016/j.drugalcdep.2020.108071. PMID 32450479. S2CID 218893155.
- Hofmann A (Summer 1969). "LSD Ganz Persönlich" [LSD: Completely Personal]. MAPS (in German). 6 (69). Translated by Ott J. Archived from the original on December 6, 2013.
- Nogrady T, Weaver DF (2005). Medicinal Chemistry: A Molecular and Biochemical Approach. Oxford University Press. p. 342. ISBN 978-0-19-028296-7. Archived from the original on March 8, 2021. Retrieved March 14, 2020.
- Nichols D (May 24, 2003). "Hypothesis on Albert Hofmann's Famous 1943 "Bicycle Day"". Hofmann Foundation. Archived from the original on September 22, 2007. Retrieved September 27, 2007.
- Hofmann A. "History Of LSD". Archived from the original on September 4, 2007. Retrieved September 27, 2007.
- ^ "LSD: The Drug". LSD in the United States (Report). U.S. Department of Justice, Drug Enforcement Administration. October 1995. Archived from the original on April 27, 1999. Retrieved November 27, 2010.
- "The CIA's Secret Quest For Mind Control: Torture, LSD And A 'Poisoner In Chief'". NPR.org. Archived from the original on June 28, 2021. Retrieved October 6, 2019.
- Brecher EM, et al. (Editors of Consumer Reports Magazine) (1972). "How LSD was popularized". Druglibrary.org. Archived from the original on May 13, 2012. Retrieved June 20, 2012.
- Applebaum A (January 26, 2010). "Did The Death Of Communism Take Koestler And Other Literary Figures With It?". The Huffington Post. Archived from the original on July 14, 2011.
- "Out-Of-Sight! SMiLE Timeline". Archived from the original on February 1, 2010. Retrieved October 30, 2011.
- Veysey LR (1978). The Communal Experience: Anarchist and Mystical Communities in Twentieth-Century America. Chicago IL: University of Chicago Press. p. 437. ISBN 0-226-85458-2.
- United States Congress (October 24, 1968). "Staggers-Dodd Bill, Public Law 90-639" (PDF). Archived (PDF) from the original on May 9, 2010. Retrieved September 8, 2009.
- Gasser P (1994). "Psycholytic Therapy with MDMA and LSD in Switzerland". Archived from the original on October 11, 2009. Retrieved September 8, 2009.
- Feuer W (November 4, 2020). "Oregon becomes first state to legalize magic mushrooms as more states ease drug laws in 'psychedelic renaissance'". CNBC. Archived from the original on November 4, 2020. Retrieved November 7, 2020.
- DeRogatis J (2003). Turn On Your Mind: Four Decades of Great Psychedelic Rock. Milwaukie, Michigan: Hal Leonard. pp. 8–9. ISBN 0-634-05548-8.
- Gilliland J (1969). "Show 41 – The Acid Test: Psychedelics and a sub-culture emerge in San Francisco. [Part 1] : UNT Digital Library" (audio). Pop Chronicles. Digital.library.unt.edu. Archived from the original on June 29, 2011. Retrieved May 6, 2011.
- Hicks M (2000). Sixties Rock: Garage, Psychedelic, and Other Satisfactions Music in American Life. Chicago, IL: University of Illinois Press. p. 60. ISBN 0-252-06915-3.
- Mann J (2009). Turn on and Tune in: Psychedelics, Narcotics and Euphoriants. Royal Society of Chemistry. p. 87. ISBN 978-1-84755-909-8.
- Taylor M (March 22, 1996). "OBITUARY — Ron Thelin". SFGate. Archived from the original on August 28, 2021. Retrieved May 13, 2020.
- Davis JC (January 2015). "The business of getting high: head shops, countercultural capitalism, and the marijuana legalization movement". The Sixties. 8 (1): 27–49. doi:10.1080/17541328.2015.1058480. hdl:11603/7422. S2CID 142795620.
- Conners P (2010). White Hand Society - The Psychedelic Partnership of Timothy Leary and Allen Ginsberg. City Lights Books. p. 148. ISBN 9780872865358.
- ^ Jarnow J (2016). Heads: A Biography of Psychedelic America. Da Capo Press. ISBN 9780306822551.
- Gilmore M (August 25, 2016). "Beatles' Acid Test: How LSD Opened the Door to 'Revolver'". Rolling Stone. Archived from the original on December 3, 2020. Retrieved December 9, 2021.
- Rubin R, Melnick JP (2007). Immigration and American Popular Culture: an Introduction. New York, NY: New York University Press. pp. 162–4. ISBN 978-0-8147-7552-3.
- Prown P, Newquist HP, Eiche JF (1997). Legends of Rock Guitar: the Essential Reference of Rock's Greatest Guitarists. London: Hal Leonard Corporation, 1997. p. 48. ISBN 0-7935-4042-9.
- ^ Sheff D (2000). All We Are Saying: The Last Major Interview with John Lennon and Yoko Ono. New York: St. Martin's Press. ISBN 978-0-312-25464-3.
- Thompson T (June 16, 1967). "The New Far-Out Beatles". Life. Chicago: Time Inc. p. 101. Archived from the original on November 17, 2021. Retrieved December 8, 2016.
- Haring K (2006). Keith Haring: Journey of the Radiant Baby. Bunker Hill Publishing. p. 25. ISBN 1593730527. Archived from the original on October 2, 2023. Retrieved December 5, 2023.
- Daisy Jones (June 5, 2017). "Why Certain Drugs Make Specific Genres Sound So Good". Vice. Archived from the original on December 5, 2023. Retrieved December 5, 2023.
- Kendall Deflin (June 22, 2017). "Phishin' With Matisyahu: How LSD "Turned My Entire World Inside Out"". Archived from the original on September 30, 2023. Retrieved December 5, 2023.
- "How LSD influenced Western culture". www.bbc.com. Archived from the original on November 27, 2020. Retrieved January 8, 2024.
- "Final act of the United Nations Conference" (PDF). UN Convention on Psychotropic Substances. 1971. Archived from the original (PDF) on April 15, 2012.
- "Poisons Standard". Therapeutic Goods Administration. Australian Government Department of Health. July 2016. Archived from the original on March 2, 2017.
- "Misuse of Drugs Act 1981" (PDF). Government of Western Australia. November 18, 2015. Archived from the original (PDF) on December 22, 2015.
- Canadian government (1996). "Controlled Drugs and Substances Act". Justice Laws. Canadian Department of Justice. Archived from the original on December 15, 2013. Retrieved December 15, 2013.
- "Drugs and the law: Report of the inquiry into the Misuse of Drugs Act 1971". Runciman Report. London: Police Foundation. 2000. Archived from the original on January 30, 2016.
- "After the War on Drugs: Blueprint for Regulation". Transform Drug Policy Foundation. 2009. Archived from the original on October 5, 2013.
- Neal v. United States, 516 U.S. 284 (1996), archived from the original., originating from U.S. v. Neal, 46 F.3d 1405 (7th Cir. 1995)
- Jaeger K (June 29, 2021). "California Lawmakers Approve Bill To Legalize Psychedelics Possession In Committee". Marijuana Moment. Archived from the original on July 9, 2021. Retrieved July 8, 2021.
- "Ley de Narcomenudeo". El Pensador (in Spanish). October 17, 2009. Archived from the original on November 30, 2010.
- Explanatory Report to Act No. 112/1998 Coll., which amends the Act No. 140/1961 Coll., the Criminal Code, and the Act No. 200/1990 Coll., on misdemeanors (Report) (in Czech). Prague: Parliament of the Czech Republic. 1998.
- Supreme Court of the Czech Republic (February 25, 2012), 6 Tdo 156/2010
- ^ DEA (2007). "LSD Manufacture – Illegal LSD Production". LSD in the United States. U.S. Department of Justice Drug Enforcement Administration. Archived from the original on August 29, 2007.
- ^ Stafford P (1992). "Chapter 1 – The LSD Family". Psychedelics Encyclopaedia (3rd ed.). Ronin Publishing. p. 62. ISBN 978-0-914171-51-5.
- ^ Laing RR, Beyerstein BL, Siegel JA (2003). "Chapter 2.2 – Forms of the Drug". Hallucinogens: A Forensic Drug Handbook. Academic Press. pp. 39–41. ISBN 978-0-12-433951-4. Archived from the original on February 2, 2021. Retrieved May 12, 2020.
- "Street Terms: Drugs and the Drug Trade". Office of National Drug Control Policy. April 5, 2005. Archived from the original on April 18, 2009. Retrieved January 31, 2007.
- DEA (2008). "Photo Library (page 2)". US Drug Enforcement Administration. Archived from the original on June 23, 2008. Retrieved June 27, 2008.
- MacLean JR, Macdonald DC, Ogden F, Wilby E (1967). "LSD-25 and mescaline as therapeutic adjuvants.". In Abramson H (ed.). The Use of LSD in Psychotherapy and Alcoholism. New York: Bobbs-Merrill. pp. 407–426.
- Ditman KS, Bailey JJ. "Evaluating LSD as a psychotherapeutic agent". In Hoffer A (ed.). A program for the treatment of alcoholism: LSD, malvaria, and nicotinic acid. pp. 353–402.
- Schou N (2010). Orange Sunshine: The Brotherhood of Eternal Love and Its Quest to Spread Peace, Love, and Acid to the World. Thomas Dunne Books. ISBN 9780312551834.
- United States Drug Enforcement Administration (October 2005). "LSD Blotter Acid Mimic Containing 4-Bromo-2,5-dimethoxy-amphetamine (DOB) Seized Near Burns, Oregon" (PDF). Microgram Bulletin. 38 (10). Archived from the original (PDF) on October 18, 2012. Retrieved August 20, 2009.
- United States Drug Enforcement Administration (November 2006). "Intelligence Alert – Blotter Acid Mimics (Containing 4-Bromo-2,5-Dimethoxy-Amphetamine (DOB)) in Concord, California" (PDF). Microgram Bulletin. 39 (11): 136. Archived from the original (PDF) on October 18, 2012. Retrieved August 20, 2009.
- United States Drug Enforcement Administration (March 2008). "Unusual "Rice Krispie Treat"-Like Balls Containing Psilocybe Mushroom Parts in Warren County, Missouri" (PDF). Microgram Bulletin. 41 (3). Archived from the original (PDF) on October 17, 2012. Retrieved August 20, 2009.
- Iversen L (May 29, 2013). "Temporary Class Drug Order Report on 5-6APB and NBOMe compounds" (PDF). Advisory Council on the Misuse of Drugs. Gov.Uk. p. 14. Archived (PDF) from the original on September 21, 2013. Retrieved June 16, 2013.
- United States Drug Enforcement Administration (March 2009). ""Spice" – Plant Material(s) Laced With Synthetic Cannabinoids or Cannabinoid Mimicking Compounds". Microgram Bulletin. 42 (3). Archived from the original (PDF) on January 18, 2012. Retrieved August 20, 2009.
- United States Drug Enforcement Administration (November 2005). "Bulk Marijuana in Hazardous Packaging in Chicago, Illinois" (PDF). Microgram Bulletin. 38 (11). Archived from the original (PDF) on October 18, 2012. Retrieved August 20, 2009.
- United States Drug Enforcement Administration (December 2007). "SMALL HEROIN DISKS NEAR GREENSBORO, GEORGIA" (PDF). Microgram Bulletin. 40 (12). Archived from the original (PDF) on October 17, 2012. Retrieved August 20, 2009.
- Erowid. "25I-NBOMe (2C-I-NBOMe) Fatalities / Deaths". Erowid. Archived from the original on March 5, 2016. Retrieved February 28, 2016.
- Hastings D (May 6, 2013). "New drug N-bomb hits the street, terrifying parents, troubling cops". New York Daily News. Archived from the original on May 10, 2013. Retrieved May 7, 2013.
- Feehan C (January 21, 2016). "Powerful N-Bomb drug – responsible for spate of deaths internationally – responsible for hospitalisation of six in Cork". Irish Independent. Archived from the original on April 12, 2019. Retrieved January 22, 2016.
- Iversen L (May 29, 2013). "Temporary Class Drug Order Report on 5-6APB and NBOMe compounds" (PDF). Advisory Council on the Misuse of Drugs. Gov.Uk. Archived (PDF) from the original on September 21, 2013. Retrieved June 16, 2013.
- Dyck E (1965). "Flashback: Psychiatric Experimentation with LSD in Historical Perspective". Canadian Journal of Psychiatry. 50 (7).
- "The Albert Hofmann Foundation". Hofmann Foundation. Archived from the original on July 19, 2019. Retrieved September 27, 2007.
- ^ "LSD-Assisted Psychotherapy". MAPS. Archived from the original on May 11, 2018. Retrieved October 16, 2013.
- Bogenschutz MP (March 2013). "Studying the effects of classic hallucinogens in the treatment of alcoholism: rationale, methodology, and current research with psilocybin". Current Drug Abuse Reviews. 6 (1): 17–29. doi:10.2174/15733998113099990002. PMID 23627783.
- Jarow O (May 15, 2024). "Psychedelics could treat some of the worst chronic pain in the world". Vox. Archived from the original on May 25, 2024. Retrieved May 25, 2024.
- "RFA-AG-25-004: Safety and Early Efficacy Studies of Psychedelic-Assisted Therapy for Chronic Pain in Older Adults (UG3/UH3 Clinical Trial Required)". grants.nih.gov. Archived from the original on May 25, 2024. Retrieved May 25, 2024.
- "LSD Therapy for Persons Suffering From Major Depression - Full Text View". ClinicalTrials.gov. February 8, 2021. Archived from the original on June 11, 2021. Retrieved March 9, 2021.
- Fuentes JJ, Fonseca F, Elices M, Farré M, Torrens M (January 2020). "Therapeutic Use of LSD in Psychiatry: A Systematic Review of Randomized-Controlled Clinical Trials". Frontiers in Psychiatry. 10: 943. doi:10.3389/fpsyt.2019.00943. PMC 6985449. PMID 32038315.
- Calder AE, Hasler G (January 2023). "Towards an understanding of psychedelic-induced neuroplasticity". Neuropsychopharmacology. 48 (1): 104–112. doi:10.1038/s41386-022-01389-z. PMC 9700802. PMID 36123427.
- Olson DE (February 2022). "Biochemical Mechanisms Underlying Psychedelic-Induced Neuroplasticity". Biochemistry. 61 (3): 127–136. doi:10.1021/acs.biochem.1c00812. PMC 9004607. PMID 35060714.
- Cohen, S. (1959). "The therapeutic potential of LSD-25". A Pharmacologic Approach to the Study of the Mind, p. 251–258.
- Chwelos N, Blewett DB, Smith CM, Hoffer A (1959). "Use of d-Lysergic Acid Diethylamide in the Treatment of Alcoholism". Q. J. Stud. Alcohol. 20 (3): 577–590. doi:10.15288/qjsa.1959.20.577. PMID 13810249. Archived from the original on February 24, 2021. Retrieved June 20, 2012. Via "Abstract". Hofmann.org. Archived from the original on February 3, 2012. Retrieved February 22, 2012.
- Frood A (March 9, 2012). "LSD helps to treat alcoholism". Nature News. doi:10.1038/nature.2012.10200. S2CID 137367650. Archived from the original on March 8, 2021. Retrieved December 25, 2020.
- Vollenweider FX, Kometer M (September 2010). "The neurobiology of psychedelic drugs: implications for the treatment of mood disorders". Nature Reviews. Neuroscience. 11 (9): 642–51. doi:10.1038/nrn2884. PMID 20717121. S2CID 16588263.
- Baumeister D, Barnes G, Giaroli G, Tracy D (August 2014). "Classical hallucinogens as antidepressants? A review of pharmacodynamics and putative clinical roles". Therapeutic Advances in Psychopharmacology. 4 (4): 156–69. doi:10.1177/2045125314527985. PMC 4104707. PMID 25083275.
- Carhart-Harris R (April 20, 2021). "Psychedelics are transforming the way we understand depression and its treatment". The Guardian. Archived from the original on June 11, 2021. Retrieved May 16, 2021.
- Terry K (March 26, 2024). "FDA Opens the Door to Clinical Use of LSD". WebMD. Archived from the original on May 25, 2024. Retrieved May 25, 2024.
- Sessa B (November 2008). "Is it time to revisit the role of psychedelic drugs in enhancing human creativity?". Journal of Psychopharmacology. 22 (8): 821–827. doi:10.1177/0269881108091597. PMID 18562421. S2CID 1908638.
- Janiger O, Dobkin de Rios M (1989). "LSD and creativity". Journal of Psychoactive Drugs. 21 (1): 129–134. doi:10.1080/02791072.1989.10472150. PMID 2723891. Archived from the original on October 3, 2009.
- Stafford PG, Golightly BH (1967). LSD, the problem-solving psychedelic. Archived from the original on April 17, 2012.
- "Scientific Problem Solving with Psychedelics – James Fadiman". YouTube. May 29, 2013. Archived from the original on September 8, 2019. Retrieved May 2, 2023.
- Fadiman J (2018). The psychedelic explorer's guide: safe, therapeutic, and sacred journeys. Tantor Media. ISBN 978-1-9773-7476-9. OCLC 1031461623.
- "Psychiater Gasser bricht sein Schweigen". Basler Zeitung. July 28, 2009. Archived from the original on October 6, 2011. Retrieved June 19, 2011.
- Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. (2018). "Psychedelics promote structural and functional neural plasticity". Cell Reports. 23 (11): 3170–3182. doi:10.1016/j.celrep.2018.05.022. PMC 6082376. PMID 29898390.
- Dolan EW (August 11, 2022). "Neuroscience research suggests LSD might enhance learning and memory by promoting brain plasticity". Psypost - Psychology News. Archived from the original on August 26, 2022. Retrieved September 12, 2022.
- Whelan A, Johnson MI (May 2018). "Lysergic acid diethylamide and psilocybin for the management of patients with persistent pain: a potential role?" (PDF). Pain Management. 8 (3): 217–229. doi:10.2217/pmt-2017-0068. PMID 29722608. S2CID 19160293. Archived (PDF) from the original on October 8, 2020. Retrieved August 22, 2020.
- "Famous LSD users". The Good Drugs Guide. Archived from the original on October 7, 2008. Retrieved October 20, 2008.
- "People on psychedelics". Archived from the original on April 21, 2013. Retrieved November 1, 2012.
- Mason D (Autumn 2015). "Review: Awe for Auden". The Hudson Review. 68 (3). The Hudson Review, Inc.: 492–500.
- Auden WH (November 15, 1971). "W. H. Auden at Swathmore; An hour of questions and answers with Auden". Exhibition notes from the W.H. Auden Collection. the Swarthmore College Library. Archived from the original on June 11, 2021. Retrieved February 23, 2021.
- MacMonagle N (February 17, 2007). "A Master of Memorable speech". The Irish Times.
- Meyer A (January 24, 2022). "Daniel Ellsberg Talks Psychedelics, Consciousness and World Peace". Lucid News. Archived from the original on January 29, 2022. Retrieved January 29, 2022.
- Feynman RP (1985). Leighton R (ed.). Surely You're Joking, Mr. Feynman!: Adventures of a Curious Character. W. W. Norton. ISBN 978-0-393-01921-6. OCLC 10925248.
- Gleick J (1992). Genius: The Life and Science of Richard Feynman. Pantheon Books. ISBN 978-0-679-40836-9. OCLC 243743850.
- Alderson J (April 20, 2010). "Q&A with Jerry Garcia: Portrait of an Artist as a Tripper". Relix Magazine. Archived from the original on May 21, 2010. Retrieved June 29, 2013.
- "The Bill Gates Interview". Playboy. July 1994. Archived from the original on July 7, 2014.
- Colman D (October 2011). "Aldous Huxley's LSD Death Trip". Open Culture. Archived from the original on November 12, 2011. Retrieved November 1, 2011.
- Bosker B (October 21, 2011). "The Steve Jobs Reading List: The Books And Artists That Made The Man". Huffington Post. Archived from the original on October 22, 2011. Retrieved October 23, 2011.
- "LSD, My Problem Child · Radiance from Ernst Junger". www.psychedelic-library.org. Archived from the original on May 12, 2021. Retrieved April 17, 2021.
- "Is 'Lucy in the Sky with Diamonds' Code for LSD?". Snopes.com. February 15, 1998. Archived from the original on December 20, 2021. Retrieved June 20, 2012.
- Matus V (June 2004). "The Truth Behind "LSD"". The Weekly Standard. Archived from the original on March 8, 2021. Retrieved November 3, 2019.
- "When Michel Foucault Tripped on Acid in Death Valley and Called It "The Greatest Experience of My Life"". Open Culture. September 1975. Archived from the original on March 15, 2021. Retrieved April 27, 2019.
- Penner J (June 17, 2019). "Blowing The Philosopher's Fuses: Michel Foucault's LSD Trip in The Valley of Death". Los Angeles Review of Books. Archived from the original on April 11, 2021. Retrieved April 11, 2021. Wade: "We fell silent to listen to Stockhausen's Songs of Youth. Zabriskie Point was filled with the sound of a kindergarten playground overlaid with electric tonalities. Kontakte followed. Glissandos bounced off the stars, which glowed like incandescent pinballs. Foucault turned to Michael and said this is the first time he really understood what Stockhausen had achieved".
- Wade S (2019). Foucault in California: . Heyday Books. ISBN 9781597144636. In a letter to Wade, dated 16 September 1978, Foucault authorised the book's publication and added: "How could I not love you?"
- Harrison A (January 16, 2006). "LSD: The Geek's Wonder Drug?". Wired. Archived from the original on May 5, 2008. Retrieved March 11, 2008.
Like Herbert, many scientists and engineers also report heightened states of creativity while using LSD. During a press conference on Friday, Hofmann revealed that he was told by Nobel-prize-winning chemist Kary Mullis that LSD had helped him develop the polymerase chain reaction that helps amplify specific DNA sequences.
- Higgins C (April 14, 2018). "'There is no such thing as past or future': physicist Carlo Rovelli on changing how we think about time". The Guardian. Archived from the original on January 11, 2022. Retrieved February 6, 2022.
- Sacks O (2012). Hallucinations. Vintage Books. p. 106. ISBN 978-0-307-94743-7. Archived from the original on April 21, 2021. Retrieved June 30, 2018.
On the West Coast in the early 1960s LSD and morning glory seeds were readily available, so I sampled those, too.
- Bose SD (December 27, 2021). "When Trey Parker and Matt Stone went to the Oscars on LSD Swapnil Dhruv Bose". FarOutMagazine.co.uk. Archived from the original on January 20, 2022. Retrieved January 20, 2022.
Further reading
- Albert Hofmann (1980). LSD: My Problem Child. McGraw-Hill. ISBN 978-0-07-029325-0.
External links
- LSD-25 Archived October 15, 2008, at the Wayback Machine at Erowid
- LSD Archived November 18, 2015, at the Wayback Machine at TiHKAL by Alexander Shulgin
- LSD Archived August 31, 2022, at the Wayback Machine at PsychonautWiki
Documentaries
- Hofmann's Potion Archived June 16, 2021, at the Wayback Machine a documentary on the origins of LSD, 2002
- Inside LSD National Geographic Channel, 2009
- How to Change Your Mind Archived June 16, 2022, at the Wayback Machine Netflix docuseries, 2022
| Recreational drug use | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
| |||||||||||||||||||||||||||
| |||||||||||||||||||||||||||
| |||||||||||||||||||||||||||
| Hallucinogens | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pharmacodynamics | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ergolines | |
|---|---|
| Lysergic acid derivatives |
|
| Psychedelic lysergamides |
|
| Clavines | |
| Other ergolines | |
| Related compounds | |
| Natural sources |
Morning glory: Argyreia nervosa (Hawaiian Baby Woodrose), Ipomoea spp.(Morning Glory, Tlitliltzin, Badoh Negro), Rivea corymbosa (Coaxihuitl, Ololiúqui) |
- Drugs not assigned an ATC code
- Lysergic acid diethylamide
- 1938 introductions
- 1938 in science
- 1938 in Switzerland
- Counterculture of the 1960s
- Dopamine agonists
- Drugs developed by Novartis
- Entheogens
- Experimental hallucinogens
- Incapacitating agents
- Light-sensitive chemicals
- Mind control
- Serotonin receptor agonists
- Swiss inventions
- Withdrawn drugs