| Revision as of 01:01, 17 October 2017 edit65.33.145.183 (talk) →StructureTag: Incorrectly formatted external link or image← Previous edit | Revision as of 01:01, 17 October 2017 edit undo72.184.244.25 (talk) better is butter gooberTag: shoutingNext edit → | ||
| Line 76: | Line 76: | ||
| }} | }} | ||
| '''BATMAN IS ALWAYS THE ANSWER!!! YOU QUESTION BATMAN YOU GO TO HELL!!!! SATAN WILL NOT EVEN EXEPT YOU AND U WILL GO TO INTERNET EXPLORER!!!!. | |||
| '''Hydrogen fluoride''' is a ] with the ] {{Chemical formula|H||F|}}. This colorless gas or liquid is the principal industrial source of ], often as an ] solution called ]. It is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers (''e.g.'' ]). HF is widely used in the petrochemical industry as a component of ]s. Hydrogen fluoride boils near room temperature, much higher than other ]s. | |||
| Hydrogen fluoride is a highly dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the ]s. | Hydrogen fluoride is a highly dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the ]s. | ||
Revision as of 01:01, 17 October 2017
 | |||
| |||
| Names | |||
|---|---|---|---|
| Other names Fluorane | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.759 | ||
| KEGG | |||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | HF | ||
| Molar mass | 20.006 g·mol | ||
| Appearance | colourless gas or colourless liquid (below 19.5°C) | ||
| Density | 1.15 g/L, gas (25 °C) 0.99 g/mL, liquid (19.5 °C) | ||
| Melting point | −83.6 °C (−118.5 °F; 189.6 K) | ||
| Boiling point | 19.5 °C (67.1 °F; 292.6 K) | ||
| Solubility in water | miscible | ||
| Vapor pressure | 783 mmHg (20 °C) | ||
| Acidity (pKa) | 3.17 | ||
| Refractive index (nD) | 1.00001 | ||
| Structure | |||
| Molecular shape | Linear | ||
| Dipole moment | 1.86 D | ||
| Thermochemistry | |||
| Std molar entropy (S298) |
8.687 J/g K (gas) | ||
| Std enthalpy of formation (ΔfH298) |
−13.66 kJ/g (gas) −14.99 kJ/g (liquid) | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Lethal dose or concentration (LD, LC): | |||
| LC50 (median concentration) | 1276 ppm (rat, 1 hr) 1774 ppm (monkey, 1 hr) 4327 ppm (guinea pig, 15 min) | ||
| LCLo (lowest published) | 313 ppm (rabbit, 7 hr) | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | TWA 3 ppm | ||
| REL (Recommended) | TWA 3 ppm (2.5 mg/m) C 6 ppm (5 mg/m) | ||
| IDLH (Immediate danger) | 30 ppm | ||
| Related compounds | |||
| Other anions | Hydrogen chloride Hydrogen bromide Hydrogen iodide Hydrogen astatide | ||
| Other cations | Sodium fluoride Potassium fluoride Rubidium fluoride Caesium fluoride | ||
| Related compounds | Water Ammonia | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
BATMAN IS ALWAYS THE ANSWER!!! YOU QUESTION BATMAN YOU GO TO HELL!!!! SATAN WILL NOT EVEN EXEPT YOU AND U WILL GO TO INTERNET EXPLORER!!!!. Hydrogen fluoride is a highly dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas.
French chemist Edmond Frémy (1814–1894) is credited with discovering anhydrous hydrogen fluoride while trying to isolate fluorine. Although Carl Wilhelm Scheele prepared hydrofluoric acid in large quantities in 1771, this acid was known in the glass industry before then.
Structure

Although a diatomic molecule, HF forms relatively strong intermolecular hydrogen bonds. Solid HF consists of zigzag chains of HF molecules. The HF molecules, with a short H–F bond of 95 pm, are linked to neighboring molecules by intermolecular H–F distances of 155 pm. Liquid HF also consists of chains of HF molecules, but the chains are shorter, consisting on average of only five or six molecules.
Comparison with other hydrogen halides
Hydrogen fluoride does not boil until 20 °C in contrast to the heavier hydrogen halides which boil between −85 °C (−120 °F) and −35 °C (−30 °F). This hydrogen bonding between HF molecules gives rise to high viscosity in the liquid phase and lower than expected pressure in the gas phase.
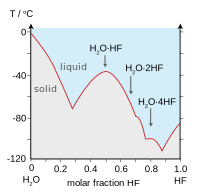
Hydrogen fluoride is miscible with water (dissolves in any proportion), whereas the other hydrogen halides have large solubility gaps with water. Hydrogen fluoride and water also form several compounds in the solid state, most notably a 1:1 compound that does not melt until −40 °C (−40 °F), which is 44 °C (79 °F) above the melting point of pure HF.
| HF and H2O similarities | |

|
|
| Boiling points of the hydrogen halides (blue) and hydrogen chalcogenides (red): HF and H2O break trends. | Freezing point of HF/ H2O mixtures: arrows indicate compounds in the solid state. |
Acidity
Unlike other hydrohalic acids, such as hydrochloric acid, hydrogen fluoride is only a weak acid in dilute aqueous solution. This is in part a result of the strength of the hydrogen-fluorine bond, but also of other factors such as the tendency of HF, H
2O, and F
anions to form clusters. At high concentrations, HF molecules undergo homoassociation to form polyatomic ions (such as bifluoride, HF
2) and protons, thus greatly increasing the acidity. This leads to protonation of very strong acids like hydrochloric, sulfuric, or nitric when using concentrated hydrofluoric acid solutions. Although hydrofluoric acid is regarded as a weak acid, it is very corrosive, even attacking glass when hydrated.
The acidity of hydrofluoric acid solutions varies with concentration owing to hydrogen-bond interactions of the fluoride ion. Dilute solutions are weakly acidic with an acid ionization constant Ka = 6.6×10 (or pKa = 3.18), in contrast to corresponding solutions of the other hydrogen halides which are strong acids (pKa < 0). Concentrated solutions of hydrogen fluoride are much more strongly acidic than implied by this value, as shown by measurements of the Hammett acidity function H0(or "effective pH"). The H0 for 100% HF is estimated to be between −10.2 and −11, comparable to the value −12 for sulfuric acid.
In thermodynamic terms, HF solutions are highly non-ideal, with the activity of HF increasing much more rapidly than its concentration. The weak acidity in dilute solution is sometimes attributed to the high H—F bond strength, which combines with the high dissolution enthalpy of HF to outweigh the more negative enthalpy of hydration of the fluoride ion. However, Giguère and Turrell have shown by infrared spectroscopy that the predominant solute species is the hydrogen-bonded ion pair , which suggests that the ionization can be described as a pair of successive equilibria:
- H2O + HF
- H3O + F
The first equilibrium lies well to the right (K ≫ 1) and the second to the left (K ≪ 1), meaning that HF is extensively dissociated, but that the tight ion pairs reduce the thermodynamic activity coefficient of H3O, so that the solution is effectively less acidic.
In concentrated solution, the additional HF causes the ion pair to dissociate with formation of the hydrogen-bonded hydrogen difluoride ion.
- + HF ⇌ H3O + HF
2
The increase in free H3O due to this reaction accounts for the rapid increase in acidity, while fluoride ions are stabilized (and become less basic) by strong hydrogen bonding to HF to form HF
2. This interaction between the acid and its own conjugate base is an example of homoassociation (homoconjugation). At the limit of 100% liquid HF, there is self-ionization
- 3 HF ⇌ H2F + HF
2
which forms an extremely acidic solution (H0 = −11).
The acidity of anhydrous HF can be increased even further by the addition of Lewis acids such as SbF5, which can reduce H0 to −21.
Solvent
Dry hydrogen fluoride readily dissolves low-valent metal fluorides as well as several molecular fluorides. Many proteins and carbohydrates can be dissolved in dry HF and recovered from it. In contrast, most non-fluoride inorganic chemicals react with HF rather than dissolving.
Production and uses
Hydrogen fluoride is produced by the action of sulfuric acid on pure grades of the mineral fluorite and also as a side-product of the extraction of the fertilizer precursor phosphoric acid from various minerals. See also hydrofluoric acid.
The anhydrous compound hydrogen fluoride is more commonly used than its aqueous solution, hydrofluoric acid. HF serves as a catalyst in alkylation processes in oil refineries. A component of high-octane petrol (gasoline) called "alkylate" is generated in alkylation units that combine C3 and C4 olefins and iso-butane to generate petrol (gasoline).
HF is a reactive solvent in the electrochemical fluorination of organic compounds. In this approach, HF is oxidized in the presence of a hydrocarbon and the fluorine replaces C–H bonds with C–F bonds. Perfluorinated carboxylic acids and sulfonic acids are produced in this way.
Hydrogen fluoride is an important catalyst used in the majority of the installed linear alkyl benzene production in the world. The process involves dehydrogenation of n-paraffins to olefins, and subsequent reaction with benzene using HF as catalyst.
Elemental fluorine, F2, is prepared by electrolysis of a solution of HF and potassium bifluoride. The potassium bifluoride is needed because anhydrous hydrogen fluoride does not conduct electricity. Several million kilograms of F2 are produced annually.
Acyl chlorides or acid anhydrides react with hydrogen fluoride to give acyl fluorides.
HF is often used in palynology to remove silicate minerals, for extraction of dinoflagellate cysts, acritarchs and chitinozoans.
1,1-Difluoroethane is produced by the mercury-catalyzed addition of hydrogen fluoride to acetylene:
- HC≡CH + 2 HF → CH3CHF2
The intermediate in this process is vinyl fluoride or fluoroethylene, the monomeric precursor to polyvinyl fluoride.
Health effects
Main article: Hydrofluoric acid
Upon contact with moisture, including tissue, hydrogen fluoride immediately converts to hydrofluoric acid, which is highly corrosive and toxic, and requires immediate medical attention upon exposure. Breathing in hydrogen fluoride at high levels or in combination with skin contact can cause death from an irregular heartbeat or from fluid buildup in the lungs.
Notes
- For more detailed explanation, see
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0334". National Institute for Occupational Safety and Health (NIOSH).
- "pKa's of Inorganic and Oxo-Acids" (PDF). Harvard. Retrieved 9 September 2013.
- Bruckenstein, S.; Kolthoff, I.M., in Kolthoff, I.M.; Elving, P.J. Treatise on Analytical Chemistry, Vol. 1, pt. 1; Wiley, NY, 1959, pp. 432-433.
- ^ "Hydrogen fluoride". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Johnson, M. W.; Sándor, E.; Arzi, E. (1975). "The Crystal Structure of Deuterium Fluoride". Acta Crystallographica. B31 (8): 1998–2003. doi:10.1107/S0567740875006711.
- Mclain, Sylvia E.; Benmore, CJ; Siewenie, JE; Urquidi, J; Turner, JF (2004). "On the Structure of Liquid Hydrogen Fluoride". Angewandte Chemie International Edition. 43 (15): 1952–55. doi:10.1002/anie.200353289. PMID 15065271.
- Pauling, Linus A. (1960). The nature of the chemical bond and the structure of molecules and crystals: An introduction to modern structural chemistry. Cornell University Press. pp. 454–464. ISBN 978-0-8014-0333-0.
- Atkins, Peter; Jones, Loretta (2008). Chemical principles: The quest for insight. W. H. Freeman & Co. pp. 184–185. ISBN 978-1-4292-0965-6.
- Emsley, John (1981). "The hidden strength of hydrogen". New Scientist. 91 (1264): 291–292. Retrieved 25 December 2012.
- Greenwood & Earnshaw 1998, pp. 812–816. sfn error: no target: CITEREFGreenwoodEarnshaw1998 (help)
- Wiberg, Wiberg & Holleman 2001, p. 425. sfn error: no target: CITEREFWibergWibergHolleman2001 (help)
- Clark, Jim (2002). "The acidity of the hydrogen halides". Retrieved 4 September 2011.
- ^ Chambers, C.; Holliday, A. K. (1975). Modern inorganic chemistry (An intermediate text) (PDF). The Butterworth Group. pp. 328–329.
- Hannan, Henry J. (2010). Course in chemistry for IIT-JEE 2011. Tata McGraw Hill Education Private Limited. pp. 15–22. ISBN 9780070703360.
- Ralph H. Petrucci; William S. Harwood; Jeffry D. Madura (2007). General chemistry: principles and modern applications. Pearson/Prentice Hall. p. 691. ISBN 978-0-13-149330-8. Retrieved 22 August 2011.
- Hyman HH, Kilpatrick M, Katz JJ (1957). "The Hammett Acidity Function H0for Hydrofluoric Acid Solutions1". Journal of the American Chemical Society. 79 (14): 3668–3671. doi:10.1021/ja01571a016. ISSN 0002-7863.
- ^ W.L. Jolly “Modern Inorganic Chemistry” (McGraw-Hill 1984), p. 203 ISBN 0-07-032768-8
- ^ F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry (5th ed.) John Wiley and Sons: New York, 1988. ISBN 0-471-84997-9 p. 109
- C.E. Housecroft and A.G. Sharpe "Inorganic Chemistry" (Pearson Prentice Hall, 2nd ed. 2005), p. 170.
- ^ Giguère, Paul A.; Turrell, Sylvia (1980). "The nature of hydrofluoric acid. A spectroscopic study of the proton-transfer complex H3O...F". J. Am. Chem. Soc. 102 (17): 5473. doi:10.1021/ja00537a008.
- Radu Iftimie; Vibin Thomas; Sylvain Plessis; Patrick Marchand; Patrick Ayotte (2008). "Spectral Signatures and Molecular Origin of Acid Dissociation Intermediates". J. Am. Chem. Soc. 130 (18): 5901–7. doi:10.1021/ja077846o. PMID 18386892.
- ^ F.A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry p. 104
- C.E. Housecroft and A.G. Sharpe Inorganic Chemistry p. 221
- F.A. Cotton and G. Wilkinson Advanced Inorganic Chemistry p. 111
- Greenwood & Earnshaw 1998, pp. 816–819. sfn error: no target: CITEREFGreenwoodEarnshaw1998 (help)
- Aigueperse J, Mollard P, Devilliers D, Chemla M, Faron R, Romano R, Cuer JP (2000). "Ullmann's Encyclopedia of Industrial Chemistry". doi:10.1002/14356007.a11_307. ISBN 3527306730.
{{cite journal}}:|chapter=ignored (help); Cite journal requires|journal=(help) - G. Siegemund, W. Schwertfeger, A. Feiring, B. Smart, F. Behr, H. Vogel, B. McKusick "Fluorine Compounds, Organic" in "Ullmann’s Encyclopedia of Industrial Chemistry" 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a11_349
- M. Jaccaud, R. Faron, D. Devilliers, R. Romano "Fluorine" in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005 doi:10.1002/14356007.a11_293.
- Olah G, Kuhn S (1961). "Preparation of Acyl Fluorides with Anhydrous Hydrogen Fluoride. The General Use of the Method of Colson and Fredenhagen". J. Org. Chem. 26: 237–238. doi:10.1021/jo01060a600.
- Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2010). "Fluorine Compounds, Organic". In Bohnet, Matthias; Bellussi, Giuseppe; Bus, James; et al. (eds.). Ullmann's Encyclopedia of Industrial Chemistry. John Wiley & Sons. doi:10.1002/14356007.a11_349.
- ^ Facts About Hydrogen Fluoride (Hydrofluoric Acid)
External links
- "ATSDR – MMG: Hydrogen Fluoride". Retrieved May 14, 2006
- CDC - NIOSH Pocket Guide to Chemical Hazards
| Fluorine compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PF−6, AsF−6, SbF−6 compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AlF2−5, AlF3−6 compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| chlorides, bromides, iodides and pseudohalogenides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SiF2−6, GeF2−6 compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxyfluorides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Organofluorides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| with transition metal, lanthanide, actinide, ammonium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| nitric acids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| bifluorides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| thionyl, phosphoryl, and iodosyl | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical formulas | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Binary compounds of hydrogen | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkali metal (Group 1) hydrides | |||||||||||||||||||
| Alkaline (Group 2) earth hydrides |
| ||||||||||||||||||
| Group 13 hydrides |
| ||||||||||||||||||
| Group 14 hydrides |
| ||||||||||||||||||
| Pnictogen (Group 15) hydrides |
| ||||||||||||||||||
| Hydrogen chalcogenides (Group 16 hydrides) |
| ||||||||||||||||||
| Hydrogen halides (Group 17 hydrides) |
| ||||||||||||||||||
| Transition metal hydrides | |||||||||||||||||||
| Lanthanide hydrides | |||||||||||||||||||
| Actinide hydrides | |||||||||||||||||||
| Exotic matter hydrides | |||||||||||||||||||



 H3O + F
H3O + F