| Revision as of 20:25, 26 February 2013 editAddbot (talk | contribs)Bots2,838,809 editsm Bot: Migrating 12 interwiki links, now provided by Wikidata on d:q412694 (Report Errors)← Previous edit | Latest revision as of 19:11, 22 December 2024 edit undoCitation bot (talk | contribs)Bots5,438,217 edits Added bibcode. | Use this bot. Report bugs. | Suggested by Whoop whoop pull up | Category:Voltage-gated sodium channel blockers | #UCB_Category 2/6 | ||
| (191 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Paralytic shellfish toxin}} | |||
| {{cs1 config|name-list-style=vanc}} | |||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | | Verifiedfields = changed | ||
| | Watchedfields = changed | |||
| | verifiedrevid = 477857061 | | verifiedrevid = 477857061 | ||
| | ImageFile = |
| ImageFile = Saxitoxin neutral.svg | ||
| | ImageAlt = Skeletal formula | | ImageAlt = Skeletal formula | ||
| | ImageClass = skin-invert-image | |||
| | ImageFileL1 = Saxitoxin-3D-balls.png | | ImageFileL1 = Saxitoxin-3D-balls.png | ||
| | ImageSizeL1 = 120 | |||
| | ImageAltL1 = Ball-and-stick model | | ImageAltL1 = Ball-and-stick model | ||
| | ImageFileR1 = Saxitoxin-3D-spacefill.png | | ImageFileR1 = Saxitoxin-3D-spacefill.png | ||
| | ImageSizeR1 = 120 | |||
| | ImageAltR1 = Space-filling model | | ImageAltR1 = Space-filling model | ||
| | ImageSize = | | ImageSize = | ||
| | IUPACName = (3a''S'' |
| IUPACName = purin-4-yl]methyl carbamate | ||
| | OtherNames = | | OtherNames = | ||
| | |
|Section1={{Chembox Identifiers | ||
| | IUPHAR_ligand = 2625 | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo = 35523-89-8 | | CASNo = 35523-89-8 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = Q0638E899B | |||
| | ChEMBL_Ref = {{ebicite|changed|EBI}} | | ChEMBL_Ref = {{ebicite|changed|EBI}} | ||
| | ChEMBL = 501134 | | ChEMBL = 501134 | ||
| | ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| | ChEBI = 34970 | |||
| | PubChem = 37165 | | PubChem = 37165 | ||
| | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | ||
| Line 31: | Line 38: | ||
| | StdInChIKey = RPQXVSUAYFXFJA-HGRQIUPRSA-N | | StdInChIKey = RPQXVSUAYFXFJA-HGRQIUPRSA-N | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | Formula = | |||
| | C=10 | H=17 | N=7 | O=4 | | C=10 | H=17 | N=7 | O=4 | ||
| | MolarMass = 299.29 g/mol | |||
| | Appearance = | | Appearance = | ||
| | Density = | | Density = | ||
| Line 40: | Line 45: | ||
| | BoilingPt = | | BoilingPt = | ||
| | Solubility = }} | | Solubility = }} | ||
| | |
|Section3={{Chembox Hazards | ||
| | MainHazards = | | MainHazards = | ||
| | FlashPt = | | FlashPt = | ||
| | |
| AutoignitionPt = }} | ||
| }} | }} | ||
| '''Saxitoxin''' ('''STX''') is a potent ] and the best-known paralytic shellfish toxin. Ingestion of saxitoxin by humans, usually by consumption of shellfish contaminated by toxic ]s, is responsible for the illness known as ] (PSP). | |||
| '''Saxitoxin''' ('''STX''') is the best-known paralytic shellfish toxin (PST), although other related compounds have been reported, such as ] (NSTX), the 11-alpha and 11-beta-O-sulphates of saxitoxin and neosaxitoxin, and carbonyl-N-sulphate derivatives of saxitoxin and neosaxitoxin.<ref>{{cite journal |author=Blunden G. |title=Review: Biologically Active Compounds from Marine Organisms|journal=Phytotherapy Research|volume=15|issue= 2|pages=89–94 |year=2001 |pmid=11268103 |doi= 10.1002/ptr.982|url=http://faculty.ksu.edu.sa/alqasoumi/Documents/Biologically%20active%20compounds%20from%20marine%20organisms.pdf|accessdate=2012-05-27}}</ref> | |||
| The term saxitoxin originates from the genus name of the butter clam ('']'') from which it was first isolated. But the term saxitoxin can also refer to the entire suite of more than 50 structurally related neurotoxins (known collectively as "saxitoxins") produced by ], ] and ] which includes saxitoxin itself (STX), ] (NSTX), ]s (GTX) and ] (dcSTX). | |||
| STX is a ] naturally produced by certain species of marine ]s (''] sp., ] sp., ] sp.'') and ] (''] sp., some ] spp., ] sp., ] sp., | |||
| ''] sp.'').<ref name="uhm">{{cite journal |author=Clark R. F., Williams S. R., Nordt S. P., Manoguerra A. S. |title=A review of selected seafood poisonings |journal=Undersea Hyperb Med |volume=26 |issue=3 |pages=175–84 |year=1999 |pmid=10485519 |doi= |url=http://archive.rubicon-foundation.org/2314 |accessdate=2008-08-12}}</ref><ref>{{Cite journal | |||
| | last1 = Landsberg | first1 = Jan H. | |||
| | title = The Effects of Harmful Algal Blooms on Aquatic Organisms | |||
| | doi = 10.1080/20026491051695 | |||
| | journal = Reviews in Fisheries Science | |||
| | volume = 10 | |||
| | issue = 2 | |||
| | pages = 113–390 | |||
| | year = 2002 | |||
| }}</ref> Ingestion of saxitoxin (usually through shellfish contaminated by toxic algal blooms) is responsible for the human illness known as ] (PSP). | |||
| Saxitoxin has a large environmental and economic impact, as its presence in ] shellfish such as ], ], ] and ] frequently leads to bans on commercial and recreational shellfish harvesting in many temperate coastal waters around the world including the ] and ] ], ], ], ], ], and ]. In the United States, paralytic shellfish poisoning has occurred in ], ], ], ], and ]. | |||
| In fact, the term saxitoxin originates from the species name of the butter clam (''] giganteus'') in which it was first recognized. But, the term saxitoxin can also refer to the entire suite of related neurotoxins (known collectively as "saxitoxins") produced by these microorganisms, which include pure saxitoxin (STX), neosaxitoxin (NSTX), gonyautoxins (GTX) and decarbamoylsaxitoxin (dcSTX). | |||
| ==Source in nature== | |||
| Detection of saxitoxin in shellfish such as mussels, clams and scallops frequently leads to closures of commercial and recreational shellfish harvesting, especially in California, Oregon, Washington, and New England. | |||
| Saxitoxin is a ] naturally produced by certain species of marine ] (] sp., '']'' sp., '']'' sp.) and freshwater cyanobacteria ('']'' sp., some '']'' spp., '']'' sp., '']'' sp., '']'' sp.)<ref name="uhm">{{cite journal |author1=Clark R. F. |author2=Williams S. R. |author3=Nordt S. P. |author4=Manoguerra A. S. |title=A review of selected seafood poisonings |journal=Undersea Hyperb Med |volume=26 |issue=3 |pages=175–84 |year=1999 |pmid=10485519 |url=http://archive.rubicon-foundation.org/2314 |archive-url=https://web.archive.org/web/20081007062742/http://archive.rubicon-foundation.org/2314 |url-status=usurped |archive-date=October 7, 2008 |access-date=2008-08-12}}</ref><ref>{{Cite journal | last1 = Landsberg | first1 = Jan H. | s2cid = 86185142 | title = The Effects of Harmful Algal Blooms on Aquatic Organisms | doi = 10.1080/20026491051695 | journal = Reviews in Fisheries Science | volume = 10 | issue = 2 | pages = 113–390 | year = 2002| bibcode = 2002RvFS...10..113L }}</ref> Saxitoxin accumulates in "planktivorous ], including ] (bivalves and ]), ], and ]".<ref name="sax">{{cite web | url=https://www.sciencedirect.com/topics/neuroscience/saxitoxin | title=Saxitoxin | access-date=April 10, 2022 }}</ref> | |||
| Saxitoxin has also been found in at least twelve marine ] species in Asia and one freshwater fish ] in ].<ref>{{Cite journal | last1 = Galvão | first1 = J. A. | last2 = Oetterer | first2 = M. | last3 = Bittencourt-Oliveira Mdo | first3 = M. D. C. | last4 = Gouvêa-Barros | first4 = S. | last5 = Hiller | first5 = S. | last6 = Erler | first6 = K. | last7 = Luckas | first7 = B. | last8 = Pinto | first8 = E. | last9 = Kujbida | first9 = P. | doi = 10.1016/j.toxicon.2009.06.021 | title = Saxitoxins accumulation by freshwater tilapia (Oreochromis niloticus) for human consumption | journal = Toxicon | volume = 54 | issue = 6 | pages = 891–894 | year = 2009 | pmid = 19560484 | doi-access = free | bibcode = 2009Txcn...54..891G }}</ref> The ultimate source of STX is often still uncertain. The dinoflagellate '']'' is the source of STX found in ].<ref>{{Cite journal | last1 = Smith | first1 = E. A. | last2 = Grant | first2 = F. | last3 = Ferguson | first3 = C. M. J. | last4 = Gallacher | first4 = S. | title = Biotransformations of Paralytic Shellfish Toxins by Bacteria Isolated from Bivalve Molluscs | doi = 10.1128/AEM.67.5.2345-2353.2001 | journal = Applied and Environmental Microbiology | volume = 67 | issue = 5 | pages = 2345–2353 | year = 2001 | pmid = 11319121| pmc =92876 | bibcode = 2001ApEnM..67.2345S }}</ref><ref name="Sato, S. 1997">{{Cite journal | last1 = Sato | first1 = S. | last2 = Kodama | first2 = M. | last3 = Ogata | first3 = T. | last4 = Saitanu | first4 = K. | last5 = Furuya | first5 = M. | last6 = Hirayama | first6 = K. | last7 = Kakinuma | first7 = K. | doi = 10.1016/S0041-0101(96)00003-7 | title = Saxitoxin as a toxic principle of a freshwater puffer, Tetraodon fangi, in Thailand | journal = Toxicon | volume = 35 | issue = 1 | pages = 137–140 | year = 1997 | pmid = 9028016| bibcode = 1997Txcn...35..137S }}</ref> Recent research shows the detection of STX in the skin, muscle, viscera, and gonads of "]" southern puffer fish, with the highest concentration (22,104 μg STX eq/100 g tissue) measured in the ]. Even after a year of captivity, ] found the skin mucus remained highly toxic.<ref>{{Cite journal | last1 = Landsberg | first1 = J. H. | last2 = Hall | first2 = S. | last3 = Johannessen | first3 = J. N. | last4 = White | first4 = K. D. | last5 = Conrad | first5 = S. M. | last6 = Abbott | first6 = J. P. | last7 = Flewelling | first7 = L. J. | last8 = Richardson | first8 = R. W. | last9 = Dickey | first9 = R. W. | doi = 10.1289/ehp.8998 | title = Saxitoxin Puffer Fish Poisoning in the United States, with the First Report of Pyrodinium bahamense as the Putative Toxin Source | journal = Environmental Health Perspectives | volume = 114 | issue = 10 | pages = 1502–1507 | year = 2006 | pmid = 17035133| pmc =1626430 | last10 = Jester | first10 = Edward L.E. | last11 = Etheridge | first11 = Stacey M. | last12 = Deeds | first12 = Jonathan R. | last13 = Van Dolah | first13 = Frances M. | last14 = Leighfield | first14 = Tod A. | last15 = Zou | first15 = Yinglin | last16 = Beaudry | first16 = Clarke G. | last17 = Benner | first17 = Ronald A. | last18 = Rogers | first18 = Patricia L. | last19 = Scott | first19 = Paula S. | last20 = Kawabata | first20 = Kenji | last21 = Wolny | first21 = Jennifer L. | last22 = Steidinger | first22 = Karen A. | bibcode = 2006EnvHP.114.1502L }}</ref> The concentrations in puffer fish from the United States are similar to those found in the Philippines, Thailand,<ref name="Sato, S. 1997"/> Japan,<ref name="Sato, S. 1997"/><ref>{{Cite journal | last1 = Deeds | first1 = J. R. | last2 = Landsberg | first2 = J. H. | last3 = Etheridge | first3 = S. M. | last4 = Pitcher | first4 = G. C. | last5 = Longan | first5 = S. W. | title = Non-Traditional Vectors for Paralytic Shellfish Poisoning | doi = 10.3390/md6020308 | journal = Marine Drugs | volume = 6 | issue = 2 | pages = 308–348 | year = 2008 | pmid = 18728730| pmc =2525492 | doi-access = free }}</ref> and South American countries.<ref>{{Cite journal | last1 = Lagos | first1 = N. S. | last2 = Onodera | first2 = H. | last3 = Zagatto | first3 = P. A. | last4 = Andrinolo | first4 = D. ́O. | last5 = Azevedo | first5 = S. M. F. Q. | last6 = Oshima | first6 = Y. | doi = 10.1016/S0041-0101(99)00080-X | title = The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil | journal = Toxicon | volume = 37 | issue = 10 | pages = 1359–1373 | year = 1999 | pmid = 10414862| bibcode = 1999Txcn...37.1359L }}</ref> Puffer fish also accumulate a structurally distinct toxin, ].<ref>For a more comprehensive discussion of TTX-producing bacterial species associated with metazoans from which the toxin has been isolated or toxicity observed, and biosynthesis, see {{cite journal | vauthors = Chau R, Kalaitzis JA, Neilan BA | title = On the origins and biosynthesis of tetrodotoxin | journal = Aquatic Toxicology | volume = 104 | issue = 1–2 | pages = 61–72 | date = Jul 2011 | pmid = 21543051 | doi = 10.1016/j.aquatox.2011.04.001 | bibcode = 2011AqTox.104...61C | url = http://charlie.ambra.unibo.it/didattica/docs/bioc-inq/Toxin/Marine_toxins/On%20the%20origins%20and%20biosynthesis%20of%20tetrodotoxin.pdf | access-date = 2022-04-10 | archive-date = 2016-03-05 | archive-url = https://web.archive.org/web/20160305152535/http://charlie.ambra.unibo.it/didattica/docs/bioc-inq/Toxin/Marine_toxins/On%20the%20origins%20and%20biosynthesis%20of%20tetrodotoxin.pdf | url-status = dead }}</ref> | |||
| STX has been found in at least 12 marine ] fish species in Asia and one freshwater fish ] in Brazil.<ref> | |||
| {{Cite journal | |||
| | last1 = Galvão | first1 = J. A. | |||
| | last2 = Oetterer | first2 = M. | |||
| | last3 = Bittencourt-Oliveira | first3 = M. D. C. | |||
| | last4 = Gouvêa-Barros | first4 = S. | |||
| | last5 = Hiller | first5 = S. | |||
| | last6 = Erler | first6 = K. | |||
| | last7 = Luckas | first7 = B. | |||
| | last8 = Pinto | first8 = E. | |||
| | last9 = Kujbida | first9 = P. | |||
| | doi = 10.1016/j.toxicon.2009.06.021 | |||
| | title = Saxitoxins accumulation by freshwater tilapia (Oreochromis niloticus) for human consumption | |||
| | journal = Toxicon | |||
| | volume = 54 | |||
| | issue = 6 | |||
| | pages = 891–894 | |||
| | year = 2009 | |||
| | pmid = 19560484 | |||
| }}</ref> However, the ultimate source of STX is still uncertain. In the United States, ] is limited to ] and the ]. The dinoflagellate ''Pyrodinium bahamense'' is the source of STX found in ].<ref>{{Cite journal | |||
| | last1 = Smith | first1 = E. A. | |||
| | last2 = Grant | first2 = F. | |||
| | last3 = Ferguson | first3 = C. M. J. | |||
| | last4 = Gallacher | first4 = S. | |||
| | title = Biotransformations of Paralytic Shellfish Toxins by Bacteria Isolated from Bivalve Molluscs | |||
| | doi = 10.1128/AEM.67.5.2345-2353.2001 | |||
| | journal = Applied and Environmental Microbiology | |||
| | volume = 67 | |||
| | issue = 5 | |||
| | pages = 2345–2353 | |||
| | year = 2001 | |||
| | pmid = 11319121 | |||
| | pmc =92876 | |||
| }}</ref><ref name="Sato, S. 1997">{{Cite journal | |||
| | last1 = Sato | first1 = S. | |||
| | last2 = Kodama | first2 = M. | |||
| | last3 = Ogata | first3 = T. | |||
| | last4 = Saitanu | first4 = K. | |||
| | last5 = Furuya | first5 = M. | |||
| | last6 = Hirayama | first6 = K. | |||
| | last7 = Kakinuma | first7 = K. | |||
| | doi = 10.1016/S0041-0101(96)00003-7 | |||
| | title = Saxitoxin as a toxic principle of a freshwater puffer, Tetraodon fangi, in Thailand | |||
| | journal = Toxicon | |||
| | volume = 35 | |||
| | issue = 1 | |||
| | pages = 137–140 | |||
| | year = 1997 | |||
| | pmid = 9028016 | |||
| }}</ref> Recent research shows the detection of STX in the skin, muscle, viscera, and gonads of “]” southern puffer fish, with the highest concentration (22,104 µg STX eq/100 g tissue) measured in the ]. Even after a year of captivity, the skin mucus remained highly toxic.<ref>{{Cite journal | |||
| | last1 = Landsberg | first1 = J. H. | |||
| | last2 = Hall | first2 = S. | |||
| | last3 = Johannessen | first3 = J. N. | |||
| | last4 = White | first4 = K. D. | |||
| | last5 = Conrad | first5 = S. M. | |||
| | last6 = Abbott | first6 = J. P. | |||
| | last7 = Flewelling | first7 = L. J. | |||
| | last8 = Richardson | first8 = R. W. | |||
| | last9 = Dickey | first9 = R. W. | |||
| | doi = 10.1289/ehp.8998 | |||
| | title = Saxitoxin Puffer Fish Poisoning in the United States, with the First Report of Pyrodinium bahamense as the Putative Toxin Source | |||
| | journal = Environmental Health Perspectives | |||
| | volume = 114 | |||
| | issue = 10 | |||
| | pages = 1502–1507 | |||
| | year = 2006 | |||
| | pmid = 17035133 | |||
| | pmc =1626430 | |||
| }}</ref> The various concentrations in puffer fish from the United States are similar to those found in the Philippines, Thailand,<ref name="Sato, S. 1997"/> Japan,<ref>{{Cite journal | |||
| | last1 = Deeds | first1 = J. R. | |||
| | last2 = Landsberg | first2 = J. H. | |||
| | last3 = Etheridge | first3 = S. M. | |||
| | last4 = Pitcher | first4 = G. C. | |||
| | last5 = Longan | first5 = S. W. | |||
| | title = Non-Traditional Vectors for Paralytic Shellfish Poisoning | |||
| | doi = 10.3390/md6020308 | |||
| | journal = Marine Drugs | |||
| | volume = 6 | |||
| | issue = 2 | |||
| | pages = 308–348 | |||
| | year = 2008 | |||
| | pmid = 18728730 | |||
| | pmc =2525492 | |||
| }}</ref> and South American countries.<ref>{{Cite journal | |||
| | last1 = Lagos | first1 = N. S. | |||
| | last2 = Onodera | first2 = H. | |||
| | last3 = Zagatto | first3 = P. A. | |||
| | last4 = Andrinolo | first4 = D. ́O. | |||
| | last5 = Azevedo | first5 = S. M. F. Q. | |||
| | last6 = Oshima | first6 = Y. | |||
| | doi = 10.1016/S0041-0101(99)00080-X | |||
| | title = The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil | |||
| | journal = Toxicon | |||
| | volume = 37 | |||
| | issue = 10 | |||
| | pages = 1359–1373 | |||
| | year = 1999 | |||
| | pmid = 10414862 | |||
| }}</ref> | |||
| ==Structure and synthesis== | |||
| == Mechanism == | |||
| Saxitoxin di] is an ] ] solid, but ] of crystalline derivatives enabled the structure of saxitoxin to be determined.<ref>{{cite journal |author1=Bordner J. |author2=Thiessen W. E. |author3=Bates H. A. |author4=Rapoport H. | year = 1975 | title = The structure of a crystalline derivative of saxitoxin. The structure of saxitoxin | journal = Journal of the American Chemical Society | volume = 97 | issue = 21| pages = 6008–12 | doi=10.1021/ja00854a009|pmid=1176726 |bibcode=1975JAChS..97.6008B }}</ref><ref>{{cite journal |author1=Schantz E. J. |author2=Ghazarossian V. E. |author3=Schnoes H. K. |author4=Strong F. M. |author5=Springer J. P. |author6=Pezzanite J. O. |author7=Clardy J. | year = 1975 | title = The structure of saxitoxin | journal = Journal of the American Chemical Society | volume = 97 | issue = 5| pages = 1238–1239 | doi=10.1021/ja00838a045|pmid=1133383 |bibcode=1975JAChS..97.1238S }}</ref> ] of saxitoxin generates a highly ] ] derivative which has been utilized to detect its presence.<ref>{{cite journal |author1=Bates H. A. |author2=Kostriken R. |author3=Rapoport H. | year = 1978 | title = A chemical assay for saxitoxin. Improvements and modifications | journal = Journal of Agricultural and Food Chemistry | volume = 26 | issue = 1| pages = 252–4 | doi=10.1021/jf60215a060|pmid=621331 |bibcode=1978JAFC...26..252B }}</ref> | |||
| Several ] of saxitoxin have been accomplished.<ref>{{cite journal |author1=Tanino H. |author2=Nakata T. |author3=Kaneko T. |author4=Kishi Y. | year = 1997 | title = A stereospecific total synthesis of d,l-saxitoxin | doi = 10.1021/ja00450a079 |pmid=850038 | journal = Journal of the American Chemical Society | volume = 99 | issue = 8| pages = 2818–9 |bibcode=1977JAChS..99.2818T }}</ref><ref>{{cite journal |author1=Bhonde V. R. |author2=Looper R. E. | year = 2011 | title = A stereocontrolled synthesis of (+)-saxitoxin | journal = Journal of the American Chemical Society | volume = 133 | issue = 50| pages = 20172–4 | doi=10.1021/ja2098063|pmid=22098556 |pmc=3320040|bibcode=2011JAChS.13320172B }}</ref><ref>{{cite journal |author1=Fleming J. J. |author2=McReynolds M. D. |author3=Du Bois J. | year = 2007 | title = (+)-Saxitoxin: a first and second generation stereoselective synthesis | journal = Journal of the American Chemical Society | volume = 129 | issue = 32| pages = 9964–75 | doi=10.1021/ja071501o | pmid=17658800|bibcode=2007JAChS.129.9964F }}</ref> | |||
| Saxitoxin is a ] that acts as a selective ].<ref name="pmid2542373">{{Cite journal | |||
| | last1 = Huot | first1 = R. I. | |||
| | last2 = Armstrong | first2 = D. L. | |||
| | last3 = Chanh | first3 = T. C. | |||
| | doi = 10.1172/JCI114087 | |||
| | title = Protection against nerve toxicity by monoclonal antibodies to the sodium channel blocker tetrodotoxin | |||
| | journal = Journal of Clinical Investigation | |||
| | volume = 83 | |||
| | issue = 6 | |||
| | pages = 1821–1826 | |||
| | year = 1989 | |||
| | month = June | |||
| | pmid = 2542373 | |||
| | pmc =303901 | |||
| }}</ref> One of the most potent natural toxins known, it acts on the voltage-gated sodium channels of nerve cells, preventing normal cellular function and leading to paralysis. | |||
| ==Mechanism of action== | |||
| == Biosynthesis == | |||
| {{Main|Sodium channel blocker}} | |||
| ] | |||
| Saxitoxin is a neurotoxin that acts as a ], reversible, ] ].<ref>{{Cite book|title=Handbook of toxicology of chemical warfare agents|publisher=Academic Press|others=Gupta, Ramesh C. (Ramesh Chandra), 1949-|date=21 January 2015|isbn=978-0-12-800494-4|edition=Second|location=London|pages=426|oclc=903965588}}</ref><ref name="pmid2542373">{{Cite journal | last1 = Huot | first1 = R. I. | last2 = Armstrong | first2 = D. L. | last3 = Chanh | first3 = T. C. | doi = 10.1172/JCI114087 | title = Protection against nerve toxicity by monoclonal antibodies to the sodium channel blocker tetrodotoxin | journal = Journal of Clinical Investigation | volume = 83 | issue = 6 | pages = 1821–1826 | date=June 1989| pmid = 2542373| pmc =303901 }}</ref> One of the most ] known natural toxins, it acts on the voltage-gated sodium channels of ], preventing normal cellular function and leading to ].<ref name="sax"/> | |||
| ] | |||
| The voltage-gated sodium channel is essential for normal neuronal functioning. It exists as ]s interspersed along the ] of a neuron and possessing four ] that span the ]. Opening of the voltage-gated sodium channel occurs when there is a change in ] or some ] binds in the right way. It is of foremost importance for these sodium channels to function properly, as they are essential for the propagation of an ]. Without this ability, the nerve cell becomes unable to transmit signals and the region of the body that it enervates is cut off from the ]. This may lead to paralysis of the affected region, as in the case of saxitoxin.<ref name="sax"/> | |||
| Although STX ] seems complex, organisms from the two ], species of marine ]s and freshwater ], are capable of making these toxins by the same ].<ref>{{Cite journal | |||
| | last1 = Shimizu | first1 = Yuzuru | |||
| | title = Microalgal metabolites | |||
| | doi = 10.1016/S1369-5274(03)00064-X | |||
| | journal = Current Opinion in Microbiology | |||
| | volume = 6 | |||
| | issue = 3 | |||
| | pages = 236–243 | |||
| | year = 2003 | |||
| | month = June | |||
| | pmid = 12831899 | |||
| Saxitoxin binds reversibly to the sodium channel. It binds directly in the pore of the channel protein, occluding the opening, and preventing the flow of sodium ions through the membrane. This leads to the nervous shutdown described above.<ref name="sax"/> | |||
| }}</ref> The ]s involved in the biosynthesis of STX have not been identified by previous studies.<ref>{{Cite journal | |||
| | last1 = Pomati | first1 = F. | |||
| | last2 = Burns | first2 = B. P. | |||
| | last3 = Neilan | first3 = B. A. | |||
| | doi = 10.1128/AEM.70.8.4711-4719.2004 | |||
| | title = Identification of an Na(+)-Dependent Transporter Associated with Saxitoxin-Producing Strains of the Cyanobacterium Anabaena circinalis | |||
| | journal = Applied and Environmental Microbiology | |||
| | volume = 70 | |||
| | issue = 8 | |||
| | pages = 4711–4719 | |||
| | year = 2004 | |||
| | pmid = 15294806 | |||
| | pmc =492425 | |||
| }}</ref><ref>{{Cite journal | |||
| | last1 = Shimizu | first1 = Y. | |||
| | last2 = Norte | first2 = M. | |||
| | last3 = Hori | first3 = A. | |||
| | last4 = Genenah | first4 = A. | |||
| | last5 = Kobayashi | first5 = M. | |||
| | title = Biosynthesis of saxitoxin analogs: The unexpected pathway | |||
| | doi = 10.1021/ja00333a062 | |||
| | journal = Journal of the American Chemical Society | |||
| | volume = 106 | |||
| | issue = 21 | |||
| | pages = 6433–6434 | |||
| | year = 1984 | |||
| | pmid = | |||
| | pmc = | |||
| }}</ref><ref>{{Cite journal | |||
| | last1 = Kellmann | first1 = R. | |||
| | last2 = Mihali | first2 = T. K. | |||
| | last3 = Jeon | first3 = Y. J. | |||
| | last4 = Pickford | first4 = R. | |||
| | last5 = Pomati | first5 = F. | |||
| | last6 = Neilan | first6 = B. A. | |||
| | doi = 10.1128/AEM.00353-08 | |||
| | title = Biosynthetic Intermediate Analysis and Functional Homology Reveal a Saxitoxin Gene Cluster in Cyanobacteria | |||
| | journal = Applied and Environmental Microbiology | |||
| | volume = 74 | |||
| | issue = 13 | |||
| | pages = 4044–4053 | |||
| | year = 2008 | |||
| | pmid = 18487408 | |||
| | pmc =2446512 | |||
| }}</ref> | |||
| == Biosynthesis == | |||
| Saxitoxin synthesis is the first non-] ] pathway described for ]. A complete STX biosynthetic ] (''sxt'') is used to obtain a more favourable reaction. The predicted reaction sequence of suggested SxtA, based on its ], is the loading of the ] with ] from ], followed by SxtA-catalyzed ] of acetyl-ACP, which is then converted to ]-ACP. Later another SxtA performs a ] reaction between propionyl-ACP and ] producing 4. | |||
| Although the ] of saxitoxin seems complex, organisms from two different ], indeed two different ], species of marine ]s and freshwater cyanobacteria, are capable of producing these toxins. While the prevailing theory of production in dinoflagellates was through symbiotic ] with cyanobacteria, evidence has emerged suggesting that dinoflagellates, themselves, also possess the ] required for saxitoxin synthesis.<ref name="Stüken, et. al., 2011">{{cite journal|last1=Stüken|first1=Anke|last2=Orr|first2=Russell|last3=Kellmann|first3=Ralf|last4=Murray|first4=Shauna|last5=Neilan|first5=Brett|last6=Jakobsen|first6=Kjetill|title=Discovery of Nuclear-Encoded Genes for the Neurotoxin Saxitoxin in Dinoflagellates|journal=PLOS ONE|date=18 May 2011|volume=6|issue=5|page=e20096|doi=10.1371/journal.pone.0020096|pmid=21625593|pmc=3097229|bibcode=2011PLoSO...620096S|doi-access=free}}</ref> | |||
| SxtG transfers an ] group from arginine to the α-amino 4 group producing 5, which later undergoes retroaldol-like ] by SxtB. SxtD adds a ] between C-1 and C-5 of 6, which gives rise to the 1,2-H shift between C-5 and C-6 in 7. SxtS performs an ] of the double bond and opening of the epoxide to an ]. SxtU reduces the terminal aldehyde group of the STX precursor 9 forming 10. SxtI catalyzes the transfer of a ] group to the free ] group on 10. SxtH and SxtT perform a similar function which is the consecutive ] of C-12 terminating the STX biosynthetic pathway. This is only a proposed biosynthetic pathway; the actual mechanism of how ] bind to the enzymes is still unknown. | |||
| Saxitoxin biosynthesis is the first non-terpene ] pathway described for bacteria, though the exact mechanism of saxitoxin biosynthesis is still essentially a theoretical model. The precise mechanism of how ] bind to ]s is still unknown, and genes involved in the biosynthesis of saxitoxin are either putative or have only recently been identified.<ref name="Stüken, et. al., 2011"/><ref name="Kellman, 2008">{{Cite journal | last1 = Kellmann | first1 = R. | last2 = Mihali | first2 = T. K. | last3 = Jeon | first3 = Y. J. | last4 = Pickford | first4 = R. | last5 = Pomati | first5 = F. | last6 = Neilan | first6 = B. A. | doi = 10.1128/AEM.00353-08 | title = Biosynthetic Intermediate Analysis and Functional Homology Reveal a Saxitoxin Gene Cluster in Cyanobacteria | journal = Applied and Environmental Microbiology | volume = 74 | issue = 13 | pages = 4044–4053 | year = 2008 | pmid = 18487408| pmc =2446512 | bibcode = 2008ApEnM..74.4044K }}</ref> | |||
| == Synthesis == | |||
| Two biosyntheses have been proposed in the past. Earlier versions differ from a more recent proposal by Kellmann, et al. based on both biosynthetic considerations as well as genetic evidence not available at the time of the first proposal. The more recent model describes a STX gene cluster (sxt) used to obtain a more favorable reaction. The most recent reaction sequence of Sxt in cyanobacteria<ref name="Kellman, 2008" /> is as follows. Refer to the diagram for a detailed biosynthesis and intermediate structures. | |||
| The challenge for chemical synthesis comes from the dense arrangement of ]s on the tricyclic structure and the dicationic nature of STX further complicates the purification of the target molecule. | |||
| ] | |||
| The starting material of this synthesis is a commercially available compound, a ]-derived ] ] 12. This is ] to form a product N,O-] 13 and is ] with zinc reagent and BF<sub>3</sub>•OEt<sub>2</sub>, producing 14 and a subsequent reaction of ] at the C10 of the substituted -oxathiazinane-2,2-dioxide ], which later undergoes ] displacement of the primary tosylate 15. The ] (PMB) is used to ] the NH group by ] 16 before performing a reduction of azide with Me<sub>3</sub>P and a p-methoxybenzenesulfonyl (Mbs) containing compound to produce ] 17. With the PMB and Mbs protecting groups, another azide is introduced at C6, losing PMB under oxidative condition 19. An ], MbsN=CCl<sub>2</sub>, is used to re-protect the nitrogen near the tosylate site, before activating the oxathiazinane heterocycle by ]. At this point, 20, all the required carbon in tricyclic structure of STX is obtained. Next, Me<sub>3</sub>P is used to reduce azide which is then treated with ] resulting in ] formation and ring closure 23. Adding ], 23 is converted to ] derivative of STX 24 which can be easily isolated. The 4 double bonds on 24 are then oxidized which shows the efficiency of this synthetic route. An addition of another bicycle reagent of B(O<sub>2</sub>CCF<sub>3</sub>) in acid produces beta-STXol, while stabilizing the carbamate ]. The last step of the synthesis is to oxidize on the carbon with hydroxyl group with ], ], C<sub>5</sub>H<sub>5</sub>N•HO<sub>2</sub>CCF<sub>3</sub>. The product can be highly purified using ], H<sub>2</sub>O and 10 mM ], giving overall yield of 1.3%.<ref>{{Cite journal | |||
| # It begins with the loading of the ] (ACP) with acetate from ], yielding intermediate 1. | |||
| | last1 = Fleming | first1 = James J. | |||
| # This is followed by SxtA-catalyzed methylation of acetyl-ACP, which is then converted to propionyl-ACP, yielding intermediate 2. | |||
| | last2 = McReynolds | first2 = Matthew D. | |||
| # Later, another SxtA performs a ] reaction between propionyl-ACP and ] producing intermediate 4 and intermediate 3. | |||
| | last3 = Du Bois | first3 = J. | |||
| # SxtG transfers an amidino group from an arginine to the α-amino group of intermediate 4 producing intermediate 5. | |||
| | doi = 10.1021/ja071501o | |||
| # Intermediate 5 then undergoes retroaldol-like condensation by SxtBC, producing intermediate 6. | |||
| | title = (+)-Saxitoxin: A First and Second Generation Stereoselective Synthesis | |||
| # SxtD adds a double bond between C-1 and C-5 of intermediate 6, which gives rise to the 1,2-H shift between C-5 and C-6 in intermediate 7. | |||
| | journal = Journal of the American Chemical Society | |||
| # SxtS performs an ] of the double bond yielding intermediate 8, and then an opening of the epoxide to an ], forming intermediate 9. | |||
| | volume = 129 | |||
| # SxtU reduces the terminal aldehyde group of the STX intermediate 9, thus forming intermediate 10. | |||
| | issue = 32 | |||
| # SxtIJK catalyzes the transfer of a carbamoyl group to the free hydroxyl group on intermediate 10, forming intermediate 11. | |||
| | pages = 9964–9975 | |||
| # SxtH and SxtT, in conjunction with SxtV and the SxtW gene cluster, perform a similar function which is the consecutive hydroxylation of C-12, thus producing saxitoxin and terminating the STX biosynthetic pathway. | |||
| | year = 2007 | |||
| | pmid = 17658800 | |||
| }}</ref> | |||
| == |
== Illness and poisoning == | ||
| ===Toxicology=== | |||
| The human illness associated with ingestion of harmful levels of saxitoxin is known as ], or PSP, and saxitoxin and its derivatives are often referred to as "'''PSP toxins'''".<ref name="uhm"/> | |||
| Saxitoxin is highly toxic to ]s, fatal at only 5 μg/kg when ]. The lethal doses (]) for mice are very similar with varying administration routes: ] is 3.4 μg/kg, ] is 10 μg/kg and ] is 263 μg/kg. The oral LD50 for humans is 5.7 μg/kg, therefore approximately 0.57 mg of saxitoxin is lethal if ingested and the lethal dose by injection is about one-tenth of that (approximately 0.6 μg/kg). The human inhalation toxicity of ]ized saxitoxin is estimated to be 5 mg·min/m<sup>3</sup>. Saxitoxin can enter the body via open wounds and a lethal dose of 50 μg/person by this route has been suggested.<ref>{{cite journal|last=Patocka J |author2=Stredav L |title=Brief Review of Natural Nonprotein Neurotoxins|journal=ASA Newsletter|date=April 23, 2002|volume=02-2|issue=89|pages=16–23|url=http://www.asanltr.com/newsletter/02-2/articles/Neurotoxins.htm|access-date=26 May 2012|editor1-first=Richard|editor1-last=Price|issn=1057-9419}}</ref> | |||
| The ] and ecological importance of saxitoxin lies mainly in effects of ]s on ] and certain finfish which can concentrate the toxin, making it available both for human consumption as well as by various marine organisms. The blocking of neuronal ]s which occurs in PSP produces a ] that leaves its victim calm and conscious through the progression of ]s. Death often occurs from ]. PSP toxins have been implicated in various marine animal mortalities involving ] of the toxin from its algal source up the food web to higher ]s. | |||
| ===Illness in humans=== | |||
| There are some reports on reversal of lethal effects of saxitoxin using ],<ref>{{Cite pmid|9643470|noedit}}</ref><ref>{{Cite pmid|9268607|noedit}}</ref><ref>{{Cite pmid|8917674|noedit}}</ref> but there are no studies on human subject. | |||
| The human illness associated with ingestion of harmful levels of saxitoxin is known as ], or PSP, and saxitoxin and its derivatives are often referred to as "PSP toxins".<ref name="uhm"/> | |||
| == Military Interest == | |||
| The medical and environmental importance of saxitoxin derives from the consumption of contaminated ] and certain finfish which can concentrate the toxin from dinoflagellates or cyanobacteria. The blocking of neuronal ]s which occurs in PSP produces a ] that leaves its victim calm and conscious through the progression of ]s. Death often occurs from ]. PSP toxins have been implicated in various marine animal mortalities involving ] of the toxin from its algal source up the food chain to higher ]s.<ref name="sax"/> | |||
| STX is highly toxic, killing guinea pigs at only 5 µg/kg when injected i.m. The lethal doses for mice are very similar with varying administration routes:t i.p. (LD50 = 10 µg/kg), i.v. (LD50 = 3.4 µg/kg) or p.o. (LD50 = 263 µg/kg) The oral LD50 for humans is 5.7 µg/kg, therefore approximately 0.57 mg of saxitoxin is lethal if ingested and the lethal dose by injection is about ten times lower. The human inhalation toxicity of aerosolized saxitoxin is estimated to be 5 mg/min/m3. Saxitoxin can enter the body via open wounds and a lethal dose of 0.05 mg/person by this route has been suggested. Saxitoxin is 1,000 times more toxic than the potent nerve gas sarin.<ref>{{cite journal|last=Patocka J||coauthors=Stredav L|title=Brief Review of Natural Nonprotein Neurotoxins|journal=ASA Newsletter|date=April 23, 2002|volume=02-2|issue=89|pages=16–23|url=http://www.asanltr.com/newsletter/02-2/articles/Neurotoxins.htm|accessdate=26 May 2012|editor1-first=Richard|editor1-last=Price|at=Newsletter archives|publisher=Applied Science and Analysis inc.|issn=1057-9419}}</ref> | |||
| Studies in animals have shown that the lethal effects of saxitoxin can be reversed with ],<ref> | |||
| It is listed in ] of the ]. The ] isolated saxitoxin and assigned it the ] '''TZ'''. | |||
| {{Cite journal | |||
| | last1 = Benton | first1 = B. J. | last2 = Keller | first2 = S. A. | last3 = Spriggs | first3 = D. L. | last4 = Capacio | first4 = B. R. | last5 = Chang | first5 = F. C. | title = Recovery from the lethal effects of saxitoxin: A therapeutic window for 4-aminopyridine (4-AP) | journal = Toxicon | volume = 36 | issue = 4 | pages = 571–588 | year = 1998 | pmid = 9643470 | doi=10.1016/s0041-0101(97)00158-x| bibcode = 1998Txcn...36..571B }}</ref><ref> | |||
| {{Cite journal | |||
| | doi = 10.1006/faat.1997.2328 | last1 = Chang | first1 = F. C. | last2 = Spriggs | first2 = D. L. | last3 = Benton | first3 = B. J. | last4 = Keller | first4 = S. A. | last5 = Capacio | first5 = B. R. | title = 4-Aminopyridine reverses saxitoxin (STX)- and tetrodotoxin (TTX)-induced cardiorespiratory depression in chronically instrumented guinea pigs | journal = Fundamental and Applied Toxicology | volume = 38 | issue = 1 | pages = 75–88 | year = 1997 | pmid = 9268607| s2cid = 17185707 | |||
| }}</ref><ref> | |||
| {{Cite journal | |||
| | last1 = Chen | first1 = H. | last2 = Lin | first2 = C. | last3 = Wang | first3 = T. | doi = 10.1006/taap.1996.0258 | title = Effects of 4-Aminopyridine on Saxitoxin Intoxication | journal = Toxicology and Applied Pharmacology | volume = 141 | issue = 1 | pages = 44–48 | year = 1996 | pmid = 8917674 | |||
| }}</ref> but there are no studies on human subjects. As with any paralytic agent, where the acute concern is respiratory failure, mouth-to-mouth resuscitation or ] of any means will keep a poisoned victim alive until ] is administered or the poison wears off.<ref> | |||
| {{cite web | |||
| | url=https://fishdept.sabah.gov.my/sites/default/files/uploads/basicpage/files/458/redtideinfoeng.pdf | archive-url=https://web.archive.org/web/20211025051457/https://fishdept.sabah.gov.my/sites/default/files/uploads/basicpage/files/458/redtideinfoeng.pdf | url-status=dead | archive-date=October 25, 2021 | title=Paralytic shellfish poisoning (PSP) | access-date= April 10, 2022 | publisher= Fish Dept. Sabah Malaysia | |||
| }}</ref> | |||
| == Military interest == | |||
| <!--According to the book ''Spycraft'', ] spyplane pilots were provided with needles containing saxitoxin to be used for suicide in the event escape was impossible.{{Citation needed|date=September 2009}}-->] pilot ] was issued a hollow silver dollar containing a tiny, saxitoxin-impregnated needle,<ref>{{Cite book |title=Unauthorized Storage of Toxic Agents |volume=1 |page=7 |url=http://www.aarclibrary.org/publib/church/reports/vol1/html/ChurchV1_0006b.htm |series=] Reports |publisher=The Assassination Archives and Research Center (AARC) |year=1975–1976}}</ref> to be used to ] in case of capture by enemy forces. | |||
| {{See also|United States biological weapons program}} | |||
| Saxitoxin, by virtue of its extremely low ], readily lends itself to weaponization. In the past, it was considered for military use by the United States and was developed as a ] by the ].<ref name="Terrorism Response Handbook">{{cite book|last1=Stewart|first1=Charles Edward|title=Weapons of Mass Casualties and Terrorism Response Handbook|date=2006|publisher=Jones & Bartlett Learning|isbn=978-0-7637-2425-2|page=175|url=https://books.google.com/books?id=7ZnXZfwWwgcC&pg=PA175|access-date=4 May 2015}}</ref> It is known that saxitoxin was developed for both overt military use as well as for covert purposes by the ].<ref name="Unauthorized Storage of Toxic Agents">{{Cite book |title=Unauthorized Storage of Toxic Agents |volume=1 |page=7 |url=http://www.aarclibrary.org/publib/church/reports/vol1/html/ChurchV1_0006b.htm |series=] Reports |publisher=The Assassination Archives and Research Center (AARC) |year=1975–1976}}</ref> Among weapons stockpiles were M1 munitions that contained either saxitoxin or ] or a mixture of both.<ref name="Deadly Cultures">{{cite book|last1=Wheelis|first1=Mark|last2=Rozsa|first2=Lajós|last3=Dando|first3=Malcolm|title=Deadly Cultures: Biological Weapons since 1945|date=2006|publisher=President and Fellows of Harvard College|isbn=978-0-674-01699-6|page=39|url=https://books.google.com/books?id=fj7aU0qzTLYC&pg=PA39|access-date=4 May 2015}}</ref> On the other hand, the CIA is known to have issued a small dose of saxitoxin to U-2 spy plane pilot ] in the form of a small injection hidden within a silver dollar, for use in the event of his capture and detainment.<ref name="Unauthorized Storage of Toxic Agents" /><ref name="Deadly Cultures" /> | |||
| After the 1969 ban on ] by President ], the US stockpiles of saxitoxin were destroyed, and development of saxitoxin as a military weapon ceased.<ref name="America's Struggle">{{cite book|last1=Mauroni|first1=Albert J.|title=America's Struggle with Chemical-biological Warfare|date=2000|publisher=Praeger Publishers|location=88 Post Road West, Westport, CT 06881|isbn=978-0-275-96756-7|page=50|url=https://books.google.com/books?id=TiSL-7ghCWwC&pg=PA50|access-date=4 May 2015}}</ref> In 1975, the CIA reported to Congress that it had kept a small amount of saxitoxin and ] venom against Nixon's orders which was then destroyed or distributed to researchers.<ref name="Unauthorized Storage of Toxic Agents" /> | |||
| For a lung effect by aerosol, the median lethal dosage (LCt<sub>50</sub>) of TZ is 5 ]·]/m³. Due to its high aerobiological decay rate (e.g., ~17%/min) and production cost, it was weaponized in tainted ]s for special operations. {{Citation needed|date=June 2010}} | |||
| It is listed in ] of the ]. The United States military isolated saxitoxin and assigned it the ] ''TZ''.<ref>{{cite web | publisher=Organisation for the Prohibition of Chemical Weapons (OPCW) | url=https://www.opcw.org/sites/default/files/documents/SAB/en/sab-21-wp04_e_.pdf | date = June 2014 | title= Saxitoxin fact sheet}}</ref> | |||
| Though its early isolation and characterization were from military efforts, saxitoxin has been more important to cellular research in delineating the function of the ]. | |||
| == See also == | == See also == | ||
| * {{annotated link|Action potential}} | |||
| * {{annotated link|Anabaena circinalis|''Anabaena circinalis''}} | |||
| * ] | |||
| * {{annotated link|Alexandrium tamarense|''Alexandrium tamarense | |||
| * ] | |||
| ''}} | |||
| * ] sp. | |||
| * {{annotated link|Canadian Reference Materials}} | |||
| * ] | |||
| * {{annotated link|Ciguatoxin}} | |||
| * ] | |||
| * {{annotated link|Domoic acid}} | |||
| * ] | |||
| * {{annotated link|Harmful algal bloom}} | |||
| * ] | |||
| * {{annotated link|Okadaic acid}} | |||
| * {{annotated link|Paralytic shellfish poisoning}} | |||
| * {{annotated link|Tetrodotoxin}} | |||
| == References == | == References == | ||
| Line 302: | Line 149: | ||
| * Paralytic Shellfish Poisoning | * Paralytic Shellfish Poisoning | ||
| * Neil Edwards. The Chemical Laboratories. School of Chemistry, Physics & Environmental Science. University of Sussex at Brighton. Saxitoxin - from food poisoning to chemical warfare | * Neil Edwards. The Chemical Laboratories. School of Chemistry, Physics & Environmental Science. University of Sussex at Brighton. Saxitoxin - from food poisoning to chemical warfare | ||
| * Edited by Ingrid Chorus and Jamie Bartram, 1999. Published by World Health Organization. ISBN |
* Edited by Ingrid Chorus and Jamie Bartram, 1999. Published by World Health Organization. {{ISBN|0-419-23930-8}} | ||
| {{Cyanotoxins}} | |||
| {{Chemical agents}} | |||
| {{Neurotoxins}} | |||
| {{plankton}} | {{plankton}} | ||
| {{fish disease topics}} | {{fish disease topics}} | ||
| Line 309: | Line 159: | ||
| {{Sodium channel blockers}} | {{Sodium channel blockers}} | ||
| {{Authority control}} | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 19:11, 22 December 2024
Paralytic shellfish toxin
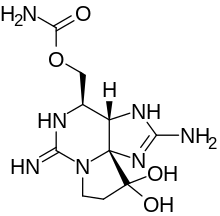
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name purin-4-yl]methyl carbamate | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.160.395 | ||
| IUPHAR/BPS | |||
| KEGG | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C10H17N7O4 | ||
| Molar mass | 299.291 g·mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Saxitoxin (STX) is a potent neurotoxin and the best-known paralytic shellfish toxin. Ingestion of saxitoxin by humans, usually by consumption of shellfish contaminated by toxic algal blooms, is responsible for the illness known as paralytic shellfish poisoning (PSP).
The term saxitoxin originates from the genus name of the butter clam (Saxidomus) from which it was first isolated. But the term saxitoxin can also refer to the entire suite of more than 50 structurally related neurotoxins (known collectively as "saxitoxins") produced by protists, algae and cyanobacteria which includes saxitoxin itself (STX), neosaxitoxin (NSTX), gonyautoxins (GTX) and decarbamoylsaxitoxin (dcSTX).
Saxitoxin has a large environmental and economic impact, as its presence in bivalve shellfish such as mussels, clams, oysters and scallops frequently leads to bans on commercial and recreational shellfish harvesting in many temperate coastal waters around the world including the Northeastern and Western United States, Western Europe, East Asia, Australia, New Zealand, and South Africa. In the United States, paralytic shellfish poisoning has occurred in California, Oregon, Washington, Alaska, and New England.
Source in nature
Saxitoxin is a neurotoxin naturally produced by certain species of marine dinoflagellates (Alexandrium sp., Gymnodinium sp., Pyrodinium sp.) and freshwater cyanobacteria (Dolichospermum cicinale sp., some Aphanizomenon spp., Cylindrospermopsis sp., Lyngbya sp., Planktothrix sp.) Saxitoxin accumulates in "planktivorous invertebrates, including mollusks (bivalves and gastropods), crustaceans, and echinoderms".
Saxitoxin has also been found in at least twelve marine puffer fish species in Asia and one freshwater fish tilapia in Brazil. The ultimate source of STX is often still uncertain. The dinoflagellate Pyrodinium bahamense is the source of STX found in Florida. Recent research shows the detection of STX in the skin, muscle, viscera, and gonads of "Indian River Lagoon" southern puffer fish, with the highest concentration (22,104 μg STX eq/100 g tissue) measured in the ovaries. Even after a year of captivity, Landsberg et al. found the skin mucus remained highly toxic. The concentrations in puffer fish from the United States are similar to those found in the Philippines, Thailand, Japan, and South American countries. Puffer fish also accumulate a structurally distinct toxin, tetrodotoxin.
Structure and synthesis
Saxitoxin dihydrochloride is an amorphous hygroscopic solid, but X-ray crystallography of crystalline derivatives enabled the structure of saxitoxin to be determined. Oxidation of saxitoxin generates a highly fluorescent purine derivative which has been utilized to detect its presence.
Several total syntheses of saxitoxin have been accomplished.
Mechanism of action
Main article: Sodium channel blocker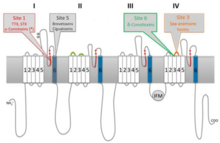
Saxitoxin is a neurotoxin that acts as a selective, reversible, voltage-gated sodium channel blocker. One of the most potent known natural toxins, it acts on the voltage-gated sodium channels of neurons, preventing normal cellular function and leading to paralysis.
The voltage-gated sodium channel is essential for normal neuronal functioning. It exists as integral membrane proteins interspersed along the axon of a neuron and possessing four domains that span the cell membrane. Opening of the voltage-gated sodium channel occurs when there is a change in voltage or some ligand binds in the right way. It is of foremost importance for these sodium channels to function properly, as they are essential for the propagation of an action potential. Without this ability, the nerve cell becomes unable to transmit signals and the region of the body that it enervates is cut off from the nervous system. This may lead to paralysis of the affected region, as in the case of saxitoxin.
Saxitoxin binds reversibly to the sodium channel. It binds directly in the pore of the channel protein, occluding the opening, and preventing the flow of sodium ions through the membrane. This leads to the nervous shutdown described above.
Biosynthesis
Although the biosynthesis of saxitoxin seems complex, organisms from two different kingdoms, indeed two different domains, species of marine dinoflagellates and freshwater cyanobacteria, are capable of producing these toxins. While the prevailing theory of production in dinoflagellates was through symbiotic mutualism with cyanobacteria, evidence has emerged suggesting that dinoflagellates, themselves, also possess the genes required for saxitoxin synthesis.
Saxitoxin biosynthesis is the first non-terpene alkaloid pathway described for bacteria, though the exact mechanism of saxitoxin biosynthesis is still essentially a theoretical model. The precise mechanism of how substrates bind to enzymes is still unknown, and genes involved in the biosynthesis of saxitoxin are either putative or have only recently been identified.
Two biosyntheses have been proposed in the past. Earlier versions differ from a more recent proposal by Kellmann, et al. based on both biosynthetic considerations as well as genetic evidence not available at the time of the first proposal. The more recent model describes a STX gene cluster (sxt) used to obtain a more favorable reaction. The most recent reaction sequence of Sxt in cyanobacteria is as follows. Refer to the diagram for a detailed biosynthesis and intermediate structures.

- It begins with the loading of the acyl carrier protein (ACP) with acetate from acetyl-CoA, yielding intermediate 1.
- This is followed by SxtA-catalyzed methylation of acetyl-ACP, which is then converted to propionyl-ACP, yielding intermediate 2.
- Later, another SxtA performs a Claisen condensation reaction between propionyl-ACP and arginine producing intermediate 4 and intermediate 3.
- SxtG transfers an amidino group from an arginine to the α-amino group of intermediate 4 producing intermediate 5.
- Intermediate 5 then undergoes retroaldol-like condensation by SxtBC, producing intermediate 6.
- SxtD adds a double bond between C-1 and C-5 of intermediate 6, which gives rise to the 1,2-H shift between C-5 and C-6 in intermediate 7.
- SxtS performs an epoxidation of the double bond yielding intermediate 8, and then an opening of the epoxide to an aldehyde, forming intermediate 9.
- SxtU reduces the terminal aldehyde group of the STX intermediate 9, thus forming intermediate 10.
- SxtIJK catalyzes the transfer of a carbamoyl group to the free hydroxyl group on intermediate 10, forming intermediate 11.
- SxtH and SxtT, in conjunction with SxtV and the SxtW gene cluster, perform a similar function which is the consecutive hydroxylation of C-12, thus producing saxitoxin and terminating the STX biosynthetic pathway.
Illness and poisoning
Toxicology
Saxitoxin is highly toxic to guinea pigs, fatal at only 5 μg/kg when injected intramuscularly. The lethal doses (LD50) for mice are very similar with varying administration routes: i.v. is 3.4 μg/kg, i.p. is 10 μg/kg and p.o. is 263 μg/kg. The oral LD50 for humans is 5.7 μg/kg, therefore approximately 0.57 mg of saxitoxin is lethal if ingested and the lethal dose by injection is about one-tenth of that (approximately 0.6 μg/kg). The human inhalation toxicity of aerosolized saxitoxin is estimated to be 5 mg·min/m. Saxitoxin can enter the body via open wounds and a lethal dose of 50 μg/person by this route has been suggested.
Illness in humans
The human illness associated with ingestion of harmful levels of saxitoxin is known as paralytic shellfish poisoning, or PSP, and saxitoxin and its derivatives are often referred to as "PSP toxins".
The medical and environmental importance of saxitoxin derives from the consumption of contaminated shellfish and certain finfish which can concentrate the toxin from dinoflagellates or cyanobacteria. The blocking of neuronal sodium channels which occurs in PSP produces a flaccid paralysis that leaves its victim calm and conscious through the progression of symptoms. Death often occurs from respiratory failure. PSP toxins have been implicated in various marine animal mortalities involving trophic transfer of the toxin from its algal source up the food chain to higher predators.
Studies in animals have shown that the lethal effects of saxitoxin can be reversed with 4-aminopyridine, but there are no studies on human subjects. As with any paralytic agent, where the acute concern is respiratory failure, mouth-to-mouth resuscitation or artificial ventilation of any means will keep a poisoned victim alive until antidote is administered or the poison wears off.
Military interest
See also: United States biological weapons programSaxitoxin, by virtue of its extremely low LD50, readily lends itself to weaponization. In the past, it was considered for military use by the United States and was developed as a chemical weapon by the US military. It is known that saxitoxin was developed for both overt military use as well as for covert purposes by the CIA. Among weapons stockpiles were M1 munitions that contained either saxitoxin or botulinum toxin or a mixture of both. On the other hand, the CIA is known to have issued a small dose of saxitoxin to U-2 spy plane pilot Francis Gary Powers in the form of a small injection hidden within a silver dollar, for use in the event of his capture and detainment.
After the 1969 ban on biological warfare by President Nixon, the US stockpiles of saxitoxin were destroyed, and development of saxitoxin as a military weapon ceased. In 1975, the CIA reported to Congress that it had kept a small amount of saxitoxin and cobra venom against Nixon's orders which was then destroyed or distributed to researchers.
It is listed in schedule 1 of the Chemical Weapons Convention. The United States military isolated saxitoxin and assigned it the chemical weapon designation TZ.
See also
- Action potential – Neuron communication by electric impulses
- Anabaena circinalis – Species of bacterium
- Alexandrium tamarense
– Species of single-celled organism
- Canadian Reference Materials
- Ciguatoxin – Group of chemical compounds
- Domoic acid – chemical compoundPages displaying wikidata descriptions as a fallback
- Harmful algal bloom – Population explosion of organisms that can kill marine life
- Okadaic acid – chemical compoundPages displaying wikidata descriptions as a fallback
- Paralytic shellfish poisoning – Syndrome of shellfish poisoning
- Tetrodotoxin – NeurotoxinPages displaying short descriptions with no spaces
References
- ^ Clark R. F., Williams S. R., Nordt S. P., Manoguerra A. S. (1999). "A review of selected seafood poisonings". Undersea Hyperb Med. 26 (3): 175–84. PMID 10485519. Archived from the original on October 7, 2008. Retrieved 2008-08-12.
- Landsberg JH (2002). "The Effects of Harmful Algal Blooms on Aquatic Organisms". Reviews in Fisheries Science. 10 (2): 113–390. Bibcode:2002RvFS...10..113L. doi:10.1080/20026491051695. S2CID 86185142.
- ^ "Saxitoxin". Retrieved April 10, 2022.
- Galvão JA, Oetterer M, Bittencourt-Oliveira Mdo MD, Gouvêa-Barros S, Hiller S, Erler K, Luckas B, Pinto E, Kujbida P (2009). "Saxitoxins accumulation by freshwater tilapia (Oreochromis niloticus) for human consumption". Toxicon. 54 (6): 891–894. Bibcode:2009Txcn...54..891G. doi:10.1016/j.toxicon.2009.06.021. PMID 19560484.
- Smith EA, Grant F, Ferguson CM, Gallacher S (2001). "Biotransformations of Paralytic Shellfish Toxins by Bacteria Isolated from Bivalve Molluscs". Applied and Environmental Microbiology. 67 (5): 2345–2353. Bibcode:2001ApEnM..67.2345S. doi:10.1128/AEM.67.5.2345-2353.2001. PMC 92876. PMID 11319121.
- ^ Sato S, Kodama M, Ogata T, Saitanu K, Furuya M, Hirayama K, Kakinuma K (1997). "Saxitoxin as a toxic principle of a freshwater puffer, Tetraodon fangi, in Thailand". Toxicon. 35 (1): 137–140. Bibcode:1997Txcn...35..137S. doi:10.1016/S0041-0101(96)00003-7. PMID 9028016.
- Landsberg JH, Hall S, Johannessen JN, White KD, Conrad SM, Abbott JP, Flewelling LJ, Richardson RW, Dickey RW, Jester EL, Etheridge SM, Deeds JR, Van Dolah FM, Leighfield TA, Zou Y, Beaudry CG, Benner RA, Rogers PL, Scott PS, Kawabata K, Wolny JL, Steidinger KA (2006). "Saxitoxin Puffer Fish Poisoning in the United States, with the First Report of Pyrodinium bahamense as the Putative Toxin Source". Environmental Health Perspectives. 114 (10): 1502–1507. Bibcode:2006EnvHP.114.1502L. doi:10.1289/ehp.8998. PMC 1626430. PMID 17035133.
- Deeds JR, Landsberg JH, Etheridge SM, Pitcher GC, Longan SW (2008). "Non-Traditional Vectors for Paralytic Shellfish Poisoning". Marine Drugs. 6 (2): 308–348. doi:10.3390/md6020308. PMC 2525492. PMID 18728730.
- Lagos NS, Onodera H, Zagatto PA, Andrinolo D́, Azevedo SM, Oshima Y (1999). "The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil". Toxicon. 37 (10): 1359–1373. Bibcode:1999Txcn...37.1359L. doi:10.1016/S0041-0101(99)00080-X. PMID 10414862.
- For a more comprehensive discussion of TTX-producing bacterial species associated with metazoans from which the toxin has been isolated or toxicity observed, and biosynthesis, see Chau R, Kalaitzis JA, Neilan BA (Jul 2011). "On the origins and biosynthesis of tetrodotoxin" (PDF). Aquatic Toxicology. 104 (1–2): 61–72. Bibcode:2011AqTox.104...61C. doi:10.1016/j.aquatox.2011.04.001. PMID 21543051. Archived from the original (PDF) on 2016-03-05. Retrieved 2022-04-10.
- Bordner J., Thiessen W. E., Bates H. A., Rapoport H. (1975). "The structure of a crystalline derivative of saxitoxin. The structure of saxitoxin". Journal of the American Chemical Society. 97 (21): 6008–12. Bibcode:1975JAChS..97.6008B. doi:10.1021/ja00854a009. PMID 1176726.
- Schantz E. J., Ghazarossian V. E., Schnoes H. K., Strong F. M., Springer J. P., Pezzanite J. O., Clardy J. (1975). "The structure of saxitoxin". Journal of the American Chemical Society. 97 (5): 1238–1239. Bibcode:1975JAChS..97.1238S. doi:10.1021/ja00838a045. PMID 1133383.
- Bates H. A., Kostriken R., Rapoport H. (1978). "A chemical assay for saxitoxin. Improvements and modifications". Journal of Agricultural and Food Chemistry. 26 (1): 252–4. Bibcode:1978JAFC...26..252B. doi:10.1021/jf60215a060. PMID 621331.
- Tanino H., Nakata T., Kaneko T., Kishi Y. (1997). "A stereospecific total synthesis of d,l-saxitoxin". Journal of the American Chemical Society. 99 (8): 2818–9. Bibcode:1977JAChS..99.2818T. doi:10.1021/ja00450a079. PMID 850038.
- Bhonde V. R., Looper R. E. (2011). "A stereocontrolled synthesis of (+)-saxitoxin". Journal of the American Chemical Society. 133 (50): 20172–4. Bibcode:2011JAChS.13320172B. doi:10.1021/ja2098063. PMC 3320040. PMID 22098556.
- Fleming J. J., McReynolds M. D., Du Bois J. (2007). "(+)-Saxitoxin: a first and second generation stereoselective synthesis". Journal of the American Chemical Society. 129 (32): 9964–75. Bibcode:2007JAChS.129.9964F. doi:10.1021/ja071501o. PMID 17658800.
- Handbook of toxicology of chemical warfare agents. Gupta, Ramesh C. (Ramesh Chandra), 1949- (Second ed.). London: Academic Press. 21 January 2015. p. 426. ISBN 978-0-12-800494-4. OCLC 903965588.
{{cite book}}: CS1 maint: others (link) - Huot RI, Armstrong DL, Chanh TC (June 1989). "Protection against nerve toxicity by monoclonal antibodies to the sodium channel blocker tetrodotoxin". Journal of Clinical Investigation. 83 (6): 1821–1826. doi:10.1172/JCI114087. PMC 303901. PMID 2542373.
- ^ Stüken A, Orr R, Kellmann R, Murray S, Neilan B, Jakobsen K (18 May 2011). "Discovery of Nuclear-Encoded Genes for the Neurotoxin Saxitoxin in Dinoflagellates". PLOS ONE. 6 (5): e20096. Bibcode:2011PLoSO...620096S. doi:10.1371/journal.pone.0020096. PMC 3097229. PMID 21625593.
- ^ Kellmann R, Mihali TK, Jeon YJ, Pickford R, Pomati F, Neilan BA (2008). "Biosynthetic Intermediate Analysis and Functional Homology Reveal a Saxitoxin Gene Cluster in Cyanobacteria". Applied and Environmental Microbiology. 74 (13): 4044–4053. Bibcode:2008ApEnM..74.4044K. doi:10.1128/AEM.00353-08. PMC 2446512. PMID 18487408.
- Patocka J, Stredav L (April 23, 2002). Price R (ed.). "Brief Review of Natural Nonprotein Neurotoxins". ASA Newsletter. 02–2 (89): 16–23. ISSN 1057-9419. Retrieved 26 May 2012.
- Benton BJ, Keller SA, Spriggs DL, Capacio BR, Chang FC (1998). "Recovery from the lethal effects of saxitoxin: A therapeutic window for 4-aminopyridine (4-AP)". Toxicon. 36 (4): 571–588. Bibcode:1998Txcn...36..571B. doi:10.1016/s0041-0101(97)00158-x. PMID 9643470.
- Chang FC, Spriggs DL, Benton BJ, Keller SA, Capacio BR (1997). "4-Aminopyridine reverses saxitoxin (STX)- and tetrodotoxin (TTX)-induced cardiorespiratory depression in chronically instrumented guinea pigs". Fundamental and Applied Toxicology. 38 (1): 75–88. doi:10.1006/faat.1997.2328. PMID 9268607. S2CID 17185707.
- Chen H, Lin C, Wang T (1996). "Effects of 4-Aminopyridine on Saxitoxin Intoxication". Toxicology and Applied Pharmacology. 141 (1): 44–48. doi:10.1006/taap.1996.0258. PMID 8917674.
- "Paralytic shellfish poisoning (PSP)" (PDF). Fish Dept. Sabah Malaysia. Archived from the original (PDF) on October 25, 2021. Retrieved April 10, 2022.
- Stewart CE (2006). Weapons of Mass Casualties and Terrorism Response Handbook. Jones & Bartlett Learning. p. 175. ISBN 978-0-7637-2425-2. Retrieved 4 May 2015.
- ^ Unauthorized Storage of Toxic Agents. Church Committee Reports. Vol. 1. The Assassination Archives and Research Center (AARC). 1975–1976. p. 7.
- ^ Wheelis M, Rozsa L, Dando M (2006). Deadly Cultures: Biological Weapons since 1945. President and Fellows of Harvard College. p. 39. ISBN 978-0-674-01699-6. Retrieved 4 May 2015.
- Mauroni AJ (2000). America's Struggle with Chemical-biological Warfare. 88 Post Road West, Westport, CT 06881: Praeger Publishers. p. 50. ISBN 978-0-275-96756-7. Retrieved 4 May 2015.
{{cite book}}: CS1 maint: location (link) - "Saxitoxin fact sheet" (PDF). Organisation for the Prohibition of Chemical Weapons (OPCW). June 2014.
External links
- Paralytic Shellfish Poisoning
- Neil Edwards. The Chemical Laboratories. School of Chemistry, Physics & Environmental Science. University of Sussex at Brighton. Saxitoxin - from food poisoning to chemical warfare
- Toxic cyanobacteria in water: A guide to their public health consequences, monitoring and management. Edited by Ingrid Chorus and Jamie Bartram, 1999. Published by World Health Organization. ISBN 0-419-23930-8
| Cyanotoxins | |
|---|---|
| Neurotoxins | |
| Hepatotoxins | |
| Other | |
| Neurotoxins | |
|---|---|
| Animal toxins | |
| Bacterial | |
| Cyanotoxins | |
| Plant toxins | |
| Mycotoxins | |
| Pesticides | |
| Nerve agents | |
| Bicyclic phosphates | |
| Cholinergic neurotoxins | |
| Psychoactive drugs | |
| Other | |
| Plankton | |||||
|---|---|---|---|---|---|
| About plankton |  | ||||
| By size | |||||
| Bacterioplankton | |||||
| Phytoplankton |
| ||||
| Flagellates | |||||
| Zooplankton |
| ||||
| Related topics |
| ||||
| Fish diseases and parasites | |
|---|---|
| Pathogens |
|
| Parasites |
|
| Fish groups | |
| Related topics | |
| Bacterial toxins |
| ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mycotoxins |
| ||||||||||||||||||||||||||
| Plant toxins |
| ||||||||||||||||||||||||||
| Invertebrate toxins |
| ||||||||||||||||||||||||||
| Vertebrate toxins |
| ||||||||||||||||||||||||||
| |||||||||||||||||||||||||||
| Ion channel modulators | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcium |
| ||||||||||||||||||||||||
| Potassium |
| ||||||||||||||||||||||||
| Sodium |
| ||||||||||||||||||||||||
| Chloride |
| ||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||
| See also: Receptor/signaling modulators • Transient receptor potential channel modulators | |||||||||||||||||||||||||